Chemistry:Mipafox
From HandWiki
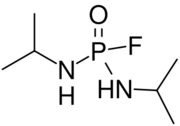
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N′-Di(propan-2-yl)phosphorodiamidic fluoride | |
| Other names
Bis(isopropylamino)fluorophosphine oxide; Isopestox
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H16FN2OP | |
| Molar mass | 182.179 g·mol−1 |
| Density | 1.2 |
| Melting point | 65 °C (149 °F; 338 K) |
| Boiling point | 125 °C (257 °F; 398 K) |
| 80 g/L | |
| Hazards | |
| Main hazards | Highly toxic |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H301, H370 | |
| P260, P264, P270, P301+310, P307+311, P321, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Mipafox is a highly toxic organophosphate insecticide that is an irreversible acetylcholinesterase inhibitor and is resistant to cholinesterase reactivators.[1] It was developed in the 1950s and is now believed to be no longer in use.[2]
Toxicity
There are case reports of delayed neurotoxicity and paralysis due to acute exposure to mipafox.[3]
Synthesis
Phosphoryl chloride is first reacted with isopropylamine. The resulting product is then reacted with potassium fluoride or ammonium fluoride to produce mipafox.[4]
See also
References
- ↑ Mangas, I; Taylor, P; Vilanova, E; Estévez, J; França, TC; Komives, E; Radić, Z (March 2016). "Resolving pathways of interaction of mipafox and a sarin analog with human acetylcholinesterase by kinetics, mass spectrometry and molecular modeling approaches.". Archives of Toxicology 90 (3): 603–16. doi:10.1007/s00204-015-1481-1. PMID 25743373.
- ↑ "The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019". World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/332193/9789240005662-eng.pdf.
- ↑ BIDSTRUP, PL; BONNELL, JA; BECKETT, AG (16 May 1953). "Paralysis following poisoning by a new organic phosphorus insecticide (mipafox); report on two cases.". British Medical Journal 1 (4819): 1068–72. doi:10.1136/bmj.1.4819.1068. PMID 13042137.
- ↑ "Process for the preparation of bisisopropyl-amino-fluoro-phosphine oxide". https://patents.google.com/patent/US2678334A.
 |
