Chemistry:Zinc carbonate
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 9157 |
| |
| |
| Properties | |
| ZnCO3 | |
| Molar mass | 125.4 |
| Appearance | white solid |
| Density | 4.434 g/cm3[1] |
| Melting point | 140 °C (284 °F; 413 K)[1] (decomposes) |
| 0.91 mg/L[1] | |
Solubility product (Ksp)
|
1.46×10−10[2] |
| -34×10−6 cm3/mol[3] | |
Refractive index (nD)
|
n1=1.621, n2=1.848[4] |
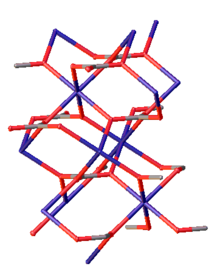
| Structure[5] | |
| Calcite, hR30, No. 167 | |
| R3c | |
a = 4.6528 Å, c = 15.025 Å
| |
Formula units (Z)
|
6 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H319, H410, H411 | |
| P264, P273, P280, P302+352, P305+351+338, P321, P332+313, P337+313, P362, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zinc carbonate is the inorganic compound with the formula ZnCO3. It is a white solid that is insoluble in water. It exists in nature as the mineral smithsonite. It is prepared by treating cold solutions of zinc sulfate with potassium bicarbonate. Upon warming, it converts to basic zinc carbonate (Zn5(CO3)2(OH)6).[6]
Zinc carbonate adopts the same structure as calcium carbonate (calcite).[7] Zinc is octahedral and each carbonate is bonded to six Zn centers such that oxygen atoms are three-coordinate.
References
- ↑ 1.0 1.1 1.2 Haynes, p. 4.95
- ↑ Haynes, p. 5.178
- ↑ Haynes, p. 4.131
- ↑ Haynes, p. 4.137
- ↑ Haynes, p. 4.144
- ↑ Wagenknecht, F.; Juza, R. (1963). "Zinc carbonate". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 2. NY, NY: Academic Press. pp. 1086.
- ↑ Effenberger, H.; Mereiter, K.; Zemann, J. (1981). "Crystal structure refinements of magnesite, calcite, rhodochrosite, siderite, smithonite, and dolomite, with discussion of some aspects of the stereochemistry of calcite type carbonates". Zeitschrift für Kristallographie - Crystalline Materials 156 (3–4): 233–243. doi:10.1524/zkri.1981.156.3-4.233. Bibcode: 1981ZK....156..233E.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
| H2CO3 | He | ||||||||||||||||
| Li2CO3, LiHCO3 |
BeCO3 | B | C | (NH4)2CO3, NH4HCO3 |
O | F | Ne | ||||||||||
| Na2CO3, NaHCO3, Na3H(CO3)2 |
MgCO3, Mg(HCO3)2 |
Al2(CO3)3 | Si | P | S | Cl | Ar | ||||||||||
| K2CO3, KHCO3 |
CaCO3, Ca(HCO3)2 |
Sc | Ti | V | Cr | MnCO3 | FeCO3 | CoCO3 | NiCO3 | CuCO3 | ZnCO3 | Ga | Ge | As | Se | Br | Kr |
| Rb2CO3 | SrCO3 | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag2CO3 | CdCO3 | In | Sn | Sb | Te | I | Xe |
| Cs2CO3, CsHCO3 |
BaCO3 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl2CO3 | PbCO3 | (BiO)2CO3 | Po | At | Rn | |
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La2(CO3)3 | Ce2(CO3)3 | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | Th | Pa | UO2CO3 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |

