Medicine:Proton-pump inhibitor
| Proton-pump inhibitor | |
|---|---|
| Drug class | |
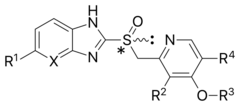 General structure of a proton-pump inhibitor | |
| Class identifiers | |
| Use | Reduction of gastric acid production |
| ATC code | A02BC |
| Mechanism of action | Enzyme inhibitor |
| Biological target | H+/K+ ATPase |
| Clinical data | |
| Drugs.com | Drug Classes |
| WebMD | MedicineNet |
| External links | |
| MeSH | D054328 |
Proton-pump inhibitors (PPIs) are a class of medications that cause a profound and prolonged reduction of stomach acid production. They do so by irreversibly inhibiting the stomach's H+/K+ ATPase proton pump.[1]
They are the most potent inhibitors of acid secretion available.[2] Proton-pump inhibitors have largely superseded the H2-receptor antagonists, a group of medications with similar effects but a different mode of action, and antacids.[3]
PPIs are among the most widely sold medications in the world. The class of proton-pump inhibitor medications is on the World Health Organization's List of Essential Medicines.[4][5] Omeprazole is the specific listed example.[4][5]
Medical uses
These medications are used in the treatment of many conditions, such as:
- Dyspepsia[6][7]
- Peptic ulcer disease including after endoscopic treatment for bleeding[8]
- As part of Helicobacter pylori eradication therapy[9]
- Gastroesophageal reflux disease (GERD or GORD) including symptomatic endoscopy-negative reflux disease[10] and associated laryngopharyngeal reflux causing laryngitis[11] and chronic cough[12]
- Barrett's esophagus[13]
- Eosinophilic esophagitis[14]
- Stress gastritis and ulcer prevention in critical care[15]
- Gastrinomas
- Zollinger–Ellison syndrome (often 2–3x the regular dose is required)[16]
Specialty professional organizations recommend that people take the lowest effective PPI dose to achieve the desired therapeutic result when used to treat gastroesophageal reflux disease long-term.[17][18][19] In the United States, the Food and Drug Administration (FDA) has advised that over-the-counter PPIs, such as Prilosec OTC, should be used no more than three 14-day treatment courses over one year.[20][21]
Despite their extensive use, the quality of the evidence supporting their use in some of these conditions is variable. The effectiveness of PPIs has not been demonstrated for every case. For example, although they reduce the incidence of esophageal adenocarcinoma in Barrett's oesophagus,[13] they do not change the length affected.[22] In addition, research in the UK has suggested that PPIs are not effective at treating persistent throat symptoms.[23][24]
Indications for stopping PPIs
PPIs are often used longer than necessary. In about half of people who are hospitalized or seen at a primary care clinic there is no documented reason for their long-term use of PPIs.[25] Some researchers believe that, given the little evidence of long-term effectiveness, the cost of the medication and the potential for harm means that clinicians should consider stopping PPIs in many people.[26]
Adverse effects
In general, proton pump inhibitors are well tolerated, and the incidence of short-term adverse effects is relatively low. The range and occurrence of adverse effects are similar for all of the PPIs, though they have been reported more frequently with omeprazole. This may be due to its longer availability and, hence, clinical experience.[citation needed]
Common adverse effects include headache, nausea, diarrhea, abdominal pain, fatigue, and dizziness.[27] Infrequent adverse effects include rash, itch, flatulence, constipation, anxiety, and depression. Also infrequently, PPI use may be associated with occurrence of myopathies, including the serious reaction rhabdomyolysis.[28]
Long-term use of PPIs requires assessment of the balance of the benefits and risks of the therapy.[29][30][31][32] As of March 2017 various adverse outcomes have been associated with long-term PPI use in several primary reports, but reviews assess the overall quality of evidence in these studies as "low" or "very low".[31] They describe inadequate evidence to establish causal relationships between PPI therapy and many of the proposed associations, due to study design and small estimates of effect size.[32] As of March 2017 Benefits outweighed risks when PPIs are used appropriately, but when used inappropriately, modest risks become important.[31][33] They recommend that PPIs should be used at the lowest effective dose in people with a proven indication, but discourage dose escalation and continued chronic therapy in people unresponsive to initial empiric therapy.[32]
Nutritional
Gastric acid is important for breakdown of food and release of micronutrients, and some studies have shown possibilities for interference with absorption of iron, calcium, magnesium, and vitamin B12.[34] With regard to iron and vitamin B12, the data are weak and several confounding factors have been identified.[30][34]
Low levels of magnesium can be found in people on PPI therapy and these can be reversed when they are switched to H2-receptor antagonist medications.[30][35][21]
Bone fractures
High dose or long-term use of PPIs carries an increased risk of bone fractures which was not found with short-term, low dose use; the FDA included a warning regarding this on PPI drug labels in 2010.[20]
In infants, acid suppression therapy is frequently prescribed to treat symptomatic gastroesophageal reflux in otherwise healthy infants (that is: without gastroesophageal reflux disease). A study from 2019 showed that PPI use alone and together with histamine H2-receptor antagonists was associated with an increased bone fracture hazard, which was amplified by days of use and earlier initiation of therapy.[36] The reason is not clear, increased bone break down by osteoclasts has been suggested.[37]
Gastrointestinal
Some studies have shown a correlation between use of PPIs and Clostridioides difficile infection. While the data are contradictory and controversial, the FDA had sufficient concern to include a warning about this adverse effect on the label of PPI medications.[30] Concerns have also been raised about spontaneous bacterial peritonitis (SBP) in older people taking PPIs and in people with irritable bowel syndrome taking PPIs; both types of infections arise in these populations due to underlying conditions and it is not clear if this is a class effect of PPIs.[30] PPIs may predispose an individual to developing small intestinal bacterial overgrowth or fungal overgrowth.[38][39]
In cirrhotic patients, large volume of ascites and reduced esophageal motility by varices can provoke GERD.[40][41][42] Acidic irritation, in return, may induce the rupture of varices.[43] Therefore, PPIs are often routinely prescribed for cirrhotic patients to treat GERD and prevent variceal bleeding. However, it has been recently shown that long term use of PPIs in patients with cirrhosis increases the risk of SBP and is associated with the development of clinical decompensation and liver-related death during long-term follow-up.[44]
There is evidence that PPI use alters the composition of the bacterial populations inhabiting the gut, the gut microbiota.[45] Although the mechanisms by which PPIs cause these changes are yet to be determined, they may have a role in the increased risk of bacterial infections with PPI use.[46] These infections can include Helicobacter pylori due to this species not favouring an acid environment, leading to an increased risk of ulcers and gastric cancer risk in genetically susceptible patients.[46]
PPI use in people who have received attempted H. pylori eradication may also be associated with an increased risk of gastric cancer.[47] The validity and robustness of this finding, with the lack of causality, have led to this association being questioned.[48] It is recommended that long-term PPIs should be used judiciously after considering individual's risk–benefit profile, particularly among those with history of H. pylori infection, and that further, well-designed, prospective studies are needed.[49]
Long-term use of PPIs is associated with the development of benign polyps from fundic glands (which is distinct from fundic gland polyposis); these polyps do not cause cancer and resolve when PPIs are discontinued.[30] There is concern that use of PPIs may mask gastric cancers or other serious gastric problems.[30]
PPI use has also been associated with the development of microscopic colitis.[50]
Cardiovascular
Associations of PPI use and cardiovascular events have also been widely studied but clear conclusions have not been made as these relative risks are confounded by other factors.[51][52] PPIs are commonly used in people with cardiovascular disease for gastric protection when aspirin is given for its antiplatelet actions.[51][53] An interaction between PPIs and the metabolism of the platelet inhibitor clopidogrel is known and this drug is also often used in people with cardiac disease.[54][55][19] There are associations with an increased risk of stroke, but this appears to be more likely to occur in people who already have an elevated risk.[56]
One suggested mechanism for cardiovascular effects is because PPIs bind and inhibit dimethylargininase, the enzyme that degrades asymmetric dimethylarginine (ADMA), resulting in higher ADMA levels and a decrease in bioavailable nitric oxide.[57]
Cancer
A 2022 umbrella review of 21 meta-analyses shows an association between proton-pump inhibitor use and an increased risk of four types of cancer.[58]
Other
Associations have been shown between PPI use and an increased risk of pneumonia, particularly in the 30 days after starting therapy, where it was found to be 50% higher in community use.[59][60] Other very weak associations of PPI use have been found, such as with chronic kidney disease,[61][62][63][19][64][65] dementia[66][31][67] and Hepatocellular carcinoma (HCC).[68]
As of 2016, results were derived from observational studies, it remained uncertain whether such associations were causal relationships.[31][32][69]
Mechanism of action
Proton pump inhibitors act by irreversibly blocking the hydrogen/potassium adenosine triphosphatase enzyme system (the H+/K+ ATPase, or, more commonly, the gastric proton pump) of the gastric parietal cells.[70] The proton pump is the terminal stage in gastric acid secretion, being directly responsible for secreting H+ ions into the gastric lumen, making it an ideal target for inhibiting acid secretion.[citation needed] Because the H,K-ATPase is the final step of acid secretion, an inhibitor of this enzyme is more effective than receptor antagonists in suppressing gastric acid secretion.[71] All of these drugs inhibit the gastric H,K-ATPase by covalent binding, so the duration of their effect is longer than expected from their levels in the blood.[72]
Targeting the terminal step in acid production, as well as the irreversible nature of the inhibition, results in a class of medications that are significantly more effective than H2 antagonists and reduce gastric acid secretion by up to 99%.[2]
Decreasing the acid in the stomach can aid the healing of duodenal ulcers and reduce the pain from indigestion and heartburn. However, stomach acids are needed to digest proteins, vitamin B12, calcium, and other nutrients, and too little stomach acid causes the condition hypochlorhydria.[citation needed]
The PPIs are given in an inactive form, which is neutrally charged (lipophilic) and readily crosses cell membranes into intracellular compartments (like the parietal cell canaliculus) with acidic environments. In an acid environment, the inactive drug is protonated and rearranges into its active form. As described above, the active form will covalently and irreversibly bind to the gastric proton pump, deactivating it.
In H. pylori eradication, PPIs help by increasing the stomach pH, causing the bacterium to shift out of its coccoid form which is resistant to both acids and antibiotics. PPIs also show some weaker additional effects in eradication.[73]
Pharmacokinetics
The rate of omeprazole absorption is decreased by concomitant food intake.[74] In addition, the absorption of lansoprazole and esomeprazole is decreased and delayed by food. It has been reported, however, that these pharmacokinetic effects have no significant impact on efficacy.[75][76]
In healthy humans, the half-life of PPIs is about 1 hour (9 hours for tenatoprazole), but the duration of acid inhibition is 48 hours because of irreversible binding to the H,K-ATPase.[77] All the PPIs except tenatoprazole are rapidly metabolized in the liver by CYP enzymes (mostly by CYP2C19 and 3A4).[77] Dissociation of the inhibitory complex is probably due to the effect of the endogenous antioxidant glutathione which leads to the release of omeprazole sulfide and reactivation of the enzyme.[78][79]
Examples
Medically used proton pump inhibitors:[citation needed]
- Dexlansoprazole[80]
- Esomeprazole (OTC and Rx-only in the US and Australia)[81]
- Ilaprazole (not FDA-approved (As of July 2019))
- Lansoprazole (OTC and Rx-only in the US)[80]
- Omeprazole (over-the-counter drug)[81]
- Pantoprazole[82]
- Rabeprazole[83]
There is no clear evidence that one proton pump inhibitor works better than another.[1][84]
History
PPIs were developed in the 1980s, with omeprazole being launched in 1988. Most of these medications are benzimidazole derivatives, related to omeprazole, but imidazopyridine derivatives such as tenatoprazole have also been developed.[2] Potassium-competitive inhibitors such as revaprazan reversibly block the potassium-binding site of the proton pump, acting more quickly, but are not available in most countries.[85]
Society and culture
Economics
In British Columbia, Canada the cost of the PPIs varies significantly from CA$0.13 to CA$2.38 per dose[86] while all agents in the class appear more or less equally effective.[1][84]
Regulatory approval
A comparative table of FDA-approved indications for PPIs is shown below.
| Indication | Omeprazole | Esomeprazole | Lansoprazole | Dexlansoprazole | Pantoprazole | Rabeprazole |
|---|---|---|---|---|---|---|
| Gastroesophageal reflux disease | ||||||
| Erosive esophagitis-healing | Yes | Yes | Yes | Yes | Yes | Yes |
| Erosive esophagitis-maintenance | Yes | Yes | Yes | Yes | Yes | Yes |
| Nonerosive reflux disease | Yes | Yes | Yes | Yes | No | Yes |
| Peptic ulcer disease | ||||||
| Duodenal ulcer-healing | Yes | No | Yes | No | No | Yes |
| Duodenal ulcer-maintenance | No | No | Yes | No | No | No |
| Gastric ulcer-healing | Yes | No | Yes | No | No | No |
| NSAID induced ulcer-healing | No | No | Yes | No | No | No |
| NSAID induced ulcer-prophylaxis | No | Yes | Yes | No | No | No |
| Zollinger-Ellison syndrome | Yes | Yes | Yes | No | Yes | Yes |
| Treatment of Helicobacter pylori | ||||||
| Dual therapy | Yes | No | Yes | No | No | No |
| Triple therapy | Yes | Yes | Yes | No | No | Yes |
References
- ↑ 1.0 1.1 1.2 "[99 Comparative effectiveness of proton pump inhibitors"]. Therapeutics Letter. 28 June 2016. https://www.ti.ubc.ca/2016/06/28/99-comparative-effectiveness-proton-pump-inhibitors/.
- ↑ 2.0 2.1 2.2 "Review article: the clinical pharmacology of proton pump inhibitors". Alimentary Pharmacology & Therapeutics 23 (Suppl 2): 2–8. June 2006. doi:10.1111/j.1365-2036.2006.02943.x. PMID 16700898.
- ↑ "Current Trends in the Management of Gastroesophageal Reflux Disease". Gut Liver 12 (1): 7–16. January 2018. doi:10.5009/gnl16615. PMID 28427116.
- ↑ 4.0 4.1 World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 5.0 5.1 World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "An overview: Current clinical guidelines for the evaluation, diagnosis, treatment, and management of dyspepsia". Osteopathic Family Physician 5 (2): 79–85. March–April 2013. doi:10.1016/j.osfp.2012.10.005.
- ↑ "Effects of proton-pump inhibitors on functional dyspepsia: a meta-analysis of randomized placebo-controlled trials". Clinical Gastroenterology and Hepatology 5 (2): 178–85; quiz 140. February 2007. doi:10.1016/j.cgh.2006.09.012. PMID 17174612.
- ↑ "Intermittent vs continuous proton pump inhibitor therapy for high-risk bleeding ulcers: a systematic review and meta-analysis". JAMA Internal Medicine 174 (11): 1755–62. November 2014. doi:10.1001/jamainternmed.2014.4056. PMID 25201154.
- ↑ "Optimum duration of regimens for Helicobacter pylori eradication". The Cochrane Database of Systematic Reviews 12 (12): CD008337. December 2013. doi:10.1002/14651858.CD008337.pub2. PMID 24338763.
- ↑ "Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease". The Cochrane Database of Systematic Reviews 5 (5): CD002095. May 2013. doi:10.1002/14651858.CD002095.pub5. PMID 23728637.
- ↑ "Proton pump inhibitor therapy for suspected GERD-related chronic laryngitis: a meta-analysis of randomized controlled trials". The American Journal of Gastroenterology 101 (11): 2646–54. November 2006. PMID 17037995.
- ↑ "Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux". BMJ 332 (7532): 11–7. January 2006. doi:10.1136/bmj.38677.559005.55. PMID 16330475.
- ↑ 13.0 13.1 "Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis". Gut 63 (8): 1229–37. August 2014. doi:10.1136/gutjnl-2013-305997. PMID 24221456.
- ↑ "Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis". Clinical Gastroenterology and Hepatology 14 (1): 13–22.e1. January 2016. doi:10.1016/j.cgh.2015.07.041. PMID 26247167.
- ↑ "Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis". Critical Care Medicine 41 (3): 693–705. March 2013. doi:10.1097/CCM.0b013e3182758734. PMID 23318494.
- ↑ "Zollinger-Ellison syndrome: classical considerations and current controversies". The Oncologist 19 (1): 44–50. January 2014. doi:10.1634/theoncologist.2013-0369. PMID 24319020.
- ↑ "Five Things Physicians and Patients Should Question". American Gastroenterological Association. 24 February 2015. http://www.choosingwisely.org/doctor-patient-lists/american-gastroenterological-association/.
- ↑ "American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease". Gastroenterology 135 (4): 1383–1391, 1391.e1-5. October 2008. doi:10.1053/j.gastro.2008.08.045. PMID 18789939.
- ↑ 19.0 19.1 19.2 "Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study". BMJ (Washington University School of Medicine) 365: l1580. May 2019. doi:10.1136/bmj.l1580. PMID 31147311. PMC 6538974. https://medicine.wustl.edu/news/popular-heartburn-drugs-linked-to-fatal-heart-disease-chronic-kidney-disease-stomach-cancer/. "Taking PPIs is associated with a small excess of cause specific mortality including death due to cardiovascular disease, chronic kidney disease, and upper gastrointestinal cancer. The burden was also observed in patients without an indication for PPI use.".
- ↑ 20.0 20.1 "FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors". U.S. Food and Drug Administration (FDA). 23 March 2011. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-possible-increased-risk-fractures-hip-wrist-and-spine-use-proton-pump.
- ↑ 21.0 21.1 "Low magnesium levels can be associated with long-term use of PPIs". 17 November 2009. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-low-magnesium-levels-can-be-associated-long-term-use-proton-pump.
- ↑ "Continuous treatment of Barrett's oesophagus patients with proton pump inhibitors up to 13 years: observations on regression and cancer incidence". Alimentary Pharmacology & Therapeutics 23 (6): 727–33. March 2006. doi:10.1111/j.1365-2036.2006.02825.x. PMID 16556174.
- ↑ (in en-GB) PPIs should not be prescribed for throat symptoms. 2022-01-13. doi:10.3310/alert_48810. https://evidence.nihr.ac.uk/alert/throat-symptoms-should-not-be-treated-with-ppis/. Retrieved 2022-07-06.
- ↑ Wilson, Janet A.; Stocken, Deborah D.; Watson, Gillian C.; Fouweather, Tony; McGlashan, Julian; MacKenzie, Kenneth; Carding, Paul; Karagama, Yakubu et al. (2021-01-22). "Lansoprazole for persistent throat symptoms in secondary care: the TOPPITS RCT" (in EN). Health Technology Assessment 25 (3): 1–118. doi:10.3310/hta25030. ISSN 2046-4924. PMID 33492208.
- ↑ "Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline". Canadian Family Physician 63 (5): 354–364. May 2017. PMID 28500192.
- ↑ "Canadian Cardiovascular Society and Choosing Wisely Canada: The Road to Creating a List of Five Things Physicians and Patients Should Question". Canadian Journal of Cardiology 30 (8): 949–955. August 2014. doi:10.1016/j.cjca.2014.06.010. ISSN 0828-282X.
- ↑ Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN:0-9757919-2-3[page needed]
- ↑ "Myopathy including polymyositis: a likely class adverse effect of proton pump inhibitors?". European Journal of Clinical Pharmacology 62 (6): 473–9. June 2006. doi:10.1007/s00228-006-0131-1. PMID 16758264.
- ↑ "Patterns of High-Dose and Long-Term Proton Pump Inhibitor Use: A Cross-Sectional Study in Six South Australian Residential Aged Care Services". Drugs - Real World Outcomes 6 (3): 105–113. September 2019. doi:10.1007/s40801-019-0157-1. PMID 31264165.
- ↑ 30.0 30.1 30.2 30.3 30.4 30.5 30.6 "Proton pump inhibitor therapy and potential long-term harm". Current Opinion in Endocrinology, Diabetes, and Obesity 21 (1): 3–8. February 2014. doi:10.1097/MED.0000000000000031. PMID 24310148.
- ↑ 31.0 31.1 31.2 31.3 31.4 "The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association". Gastroenterology 152 (4): 706–715. March 2017. doi:10.1053/j.gastro.2017.01.031. PMID 28257716. "Conclusions:Baseline differences between PPI users and non-users make it challenging to study potential PPI adverse effects retrospectively. Despite a large number of studies, the overall quality of evidence for PPI adverse effects is low to very low. When PPIs are appropriately prescribed, their benefits are likely to outweigh their risks. When PPIs are inappropriately prescribed, modest risks become important because there is no potential benefit. There is currently insufficient evidence to recommend specific strategies for mitigating PPI adverse effects.".
- ↑ 32.0 32.1 32.2 32.3 "Complications of Proton Pump Inhibitor Therapy". Gastroenterology 153 (1): 35–48. July 2017. doi:10.1053/j.gastro.2017.04.047. PMID 28528705. "In turn, this has caused unnecessary concern among patients and prescribers. The benefits of PPI therapy for appropriate indications need to be considered, along with the likelihood of the proposed risks. Patients with a proven indication for a PPI should continue to receive it in the lowest effective dose. PPI dose escalation and continued chronic therapy in those unresponsive to initial empiric therapy is discouraged.".
- ↑ "Regular use of proton-pump inhibitors and risk of stroke: a population-based cohort study and meta-analysis of randomized-controlled trials". BMC Medicine 19 (1): 316. December 2021. doi:10.1186/s12916-021-02180-5. PMID 34856983.
- ↑ 34.0 34.1 "Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium". Current Gastroenterology Reports 12 (6): 448–57. December 2010. doi:10.1007/s11894-010-0141-0. PMID 20882439.
- ↑ "The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis". PLOS ONE 9 (11): e112558. 2014. doi:10.1371/journal.pone.0112558. PMID 25394217. Bibcode: 2014PLoSO...9k2558P.
- ↑ Malchodi, Laura; Wagner, Kari; Susi, Apryl; Gorman, Gregory; Hisle-Gorman, Elizabeth (July 2019). "Early Acid Suppression Therapy Exposure and Fracture in Young Children". Pediatrics 144 (1): e20182625. doi:10.1542/peds.2018-2625. ISSN 1098-4275. PMID 31175146.
- ↑ Nehra, Avinash K.; Alexander, Jeffrey A.; Loftus, Conor G.; Nehra, Vandana (2018). "Proton Pump Inhibitors: Review of Emerging Concerns" (in en). Mayo Clinic Proceedings 93 (2): 240–246. doi:10.1016/j.mayocp.2017.10.022. PMID 29406201.
- ↑ "What are the effects of proton pump inhibitors on the small intestine?". World Journal of Gastroenterology 21 (22): 6817–9. June 2015. doi:10.3748/wjg.v21.i22.6817. PMID 26078557. "Generally, proton-pump inhibitors (PPIs) have great benefit for patients with acid related disease with less frequently occurring side effects. According to a recent report, PPIs provoke dysbiosis of the small intestinal bacterial flora, exacerbating nonsteroidal anti-inflammatory drug-induced small intestinal injury. Several meta-analyses and systematic reviews have reported that patients treated with PPIs, as well as post-gastrectomy patients, have a higher frequency of small intestinal bacterial overgrowth (SIBO) compared to patients who lack the aforementioned conditions. Furthermore, there is insufficient evidence that these conditions induce Clostridium difficile infection. At this time, PPI-induced dysbiosis is considered a type of SIBO.".
- ↑ "Small intestinal fungal overgrowth". Current Gastroenterology Reports 17 (4): 16. April 2015. doi:10.1007/s11894-015-0436-2. PMID 25786900. "Small intestinal fungal overgrowth (SIFO) is characterized by the presence of excessive number of fungal organisms in the small intestine associated with gastrointestinal (GI) symptoms. Candidiasis is known to cause GI symptoms particularly in immunocompromised patients or those receiving steroids or antibiotics. However, only recently, there is emerging literature that an overgrowth of fungus in the small intestine of non-immunocompromised subjects may cause unexplained GI symptoms. Two recent studies showed that 26% (24/94) and 25.3% (38/150) of a series of patients with unexplained GI symptoms had SIFO. The most common symptoms observed in these patients were belching, bloating, indigestion, nausea, diarrhea, and gas. The underlying mechanism(s) that predisposes to SIFO is unclear but small intestinal dysmotility and use of proton pump inhibitors has been implicated. However, further studies are needed; both to confirm these observations and to examine the clinical relevance of fungal overgrowth, both in healthy subjects and in patients with otherwise unexplained GI symptoms.".
- ↑ "High prevalence of reflux esophagitis among upper endoscopies in Chinese patients with chronic liver diseases". BMC Gastroenterology 10 (1): 54. June 2010. doi:10.1186/1471-230X-10-54. PMID 20525368.
- ↑ "Esophageal motility in cirrhotics with and without esophageal varices". Scandinavian Journal of Gastroenterology 24 (3): 334–8. April 1989. doi:10.3109/00365528909093056. PMID 2734592.
- ↑ "Esophageal function after injection sclerotherapy: pathogenesis of esophageal stricture". American Journal of Surgery 147 (1): 85–8. January 1984. doi:10.1016/0002-9610(84)90039-4. PMID 6606991.
- ↑ "Controlled trial of ligation plus vasoconstrictor versus proton pump inhibitor in the control of acute esophageal variceal bleeding". Journal of Gastroenterology and Hepatology 28 (4): 684–9. April 2013. doi:10.1111/jgh.12107. PMID 23278466.
- ↑ "Deleterious effect of proton pump inhibitors on the disease course of cirrhosis" (in en-US). European Journal of Gastroenterology & Hepatology 32 (2): 257–264. February 2020. doi:10.1097/MEG.0000000000001499. PMID 31464790.
- ↑ "Proton pump inhibitors alter the composition of the gut microbiota". Gut 65 (5): 749–756. May 2016. doi:10.1136/gutjnl-2015-310861. PMID 26719299.
- ↑ 46.0 46.1 "Proton pump inhibitors and helicobacter pylori-associated pathogenesis". Asian Pacific Journal of Cancer Prevention 16 (4): 1315–1319. 2015. doi:10.7314/APJCP.2015.16.4.1315. PMID 25743791.
- ↑ "Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study". Gut 67 (1): 28–35. January 2018. doi:10.1136/gutjnl-2017-314605. PMID 29089382.
- ↑ "Canadian Association of Gastroenterology Statement on the Putative Link Between Proton Pump Inhibitor Treatment and Gastric Cancer after Helicobacter pylori Eradication". Journal of the Canadian Association of Gastroenterology 1 (4): 155–158. December 2018. doi:10.1093/jcag/gwy040. PMID 31294357.
- ↑ "Long-term use of proton-pump inhibitors and risk of gastric cancer: a review of the current evidence". Therapeutic Advances in Gastroenterology 12: 1756284819834511. January 2019. doi:10.1177/1756284819834511. PMID 30886648.
- ↑ "Microscopic colitis: Current status, present and future challenges: statements of the European Microscopic Colitis Group". Journal of Crohn's & Colitis 6 (9): 932–45. October 2012. doi:10.1016/j.crohns.2012.05.014. PMID 22704658.
- ↑ 51.0 51.1 "Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy". European Heart Journal 34 (23): 1708–13, 1713a-1713b. June 2013. doi:10.1093/eurheartj/eht042. PMID 23425521.
- ↑ "Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review". Circulation: Cardiovascular Quality and Outcomes 8 (1): 47–55. January 2015. doi:10.1161/CIRCOUTCOMES.114.001177. PMID 25587094.
- ↑ "Effects of proton pump inhibitors on adverse gastrointestinal events in patients receiving clopidogrel: systematic review and meta-analysis". Drug Safety 34 (1): 47–57. January 2011. doi:10.2165/11584750-000000000-00000. PMID 21047145.
- ↑ "Concomitant use of clopidogrel and proton pump inhibitors: impact on platelet function and clinical outcome- a systematic review". Heart 99 (8): 520–7. April 2013. doi:10.1136/heartjnl-2012-302371. PMID 22851683.
- ↑ "Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: an updated meta-analysis". Open Heart 2 (1): e000248. 2015. doi:10.1136/openhrt-2015-000248. PMID 26196021.
- ↑ "Regular use of proton-pump inhibitors and risk of stroke: a population-based cohort study and meta-analysis of randomized-controlled trials". BMC Medicine (Springer Science and Business Media LLC) 19 (1): 316. December 2021. doi:10.1186/s12916-021-02180-5. PMID 34856983.
- ↑ "Dimethylarginines ADMA and SDMA: the real water-soluble small toxins?". Seminars in Nephrology 34 (2): 97–105. March 2014. doi:10.1016/j.semnephrol.2014.02.003. PMID 24780466. "It also seems to be the pathophysiological link between the use of proton pump inhibitors and increased cardiovascular event rate because these medications bind and inhibit DDAH, the enzyme that degrades ADMA, which results in higher ADMA levels and a decrease in bioavailable NO.".
- ↑ Zhang, M. L.; Fan, Y. X.; Meng, R.; Cai, W. K.; Yin, S. J.; Zhou, T.; Huang, Y. H.; Wang, P. et al. (2022). "Proton Pump Inhibitors and Cancer Risk: An Umbrella Review and Meta-analysis of Observational Studies". American Journal of Clinical Oncology 45 (11): 475–485. doi:10.1097/COC.0000000000000949. PMID 36255347.
- ↑ "Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis". PLOS ONE 10 (6): e0128004. 2015. doi:10.1371/journal.pone.0128004. PMID 26042842. Bibcode: 2015PLoSO..1028004L.
- ↑ "Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis". CMAJ 183 (3): 310–9. February 2011. doi:10.1503/cmaj.092129. PMID 21173070.
- ↑ "Proton pump inhibitors use and risk of chronic kidney disease: Evidence-based meta-analysis of observational studies". Clinical Epidemiology and Global Health 7: 46–52. 2019. doi:10.1016/j.cegh.2017.12.008.
- ↑ "Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease". JAMA Internal Medicine (American Medical Association (AMA)) 176 (2): 238–46. February 2016. doi:10.1001/jamainternmed.2015.7193. PMID 26752337.
- ↑ "Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury". Kidney International (Elsevier BV) 91 (6): 1482–1494. June 2017. doi:10.1016/j.kint.2016.12.021. PMID 28237709.
- ↑ "Proton Pump Inhibitors and CKD". Journal of the American Society of Nephrology (American Society of Nephrology (ASN)) 27 (10): 2926–2928. October 2016. doi:10.1681/asn.2016020192. PMID 27080978.
- ↑ "Proton Pump Inhibitors and Risk of Incident CKD and Progression to ESRD". Journal of the American Society of Nephrology (American Society of Nephrology (ASN)) 27 (10): 3153–3163. October 2016. doi:10.1681/asn.2015121377. PMID 27080976.
- ↑ Salman Hussain, Ambrish Singh et al. No association between proton pump inhibitors use and risk of dementia: Evidence from a meta-analysis. J Gastroenterol Hepatol. https://doi.org/10.1111/jgh.14789
- ↑ "Clinical Implications of Emerging Data on the Safety of Proton Pump Inhibitors". Current Treatment Options in Gastroenterology 15 (1): 1–9. March 2017. doi:10.1007/s11938-017-0115-5. PMID 28130652. "The methodology of these studies allows us to find an association with these events but does not provide us with sufficient evidence to determine causality. In general, the findings of the available studies do not fit with our clinical experience nor is the magnitude of the association sufficient to result in a major change in our practice. Nevertheless, the recent literature has resulted in our careful reevaluation of PPI use across both FDA indications and in general.".
- ↑ "Proton pump inhibitor use and the risk of hepatocellular carcinoma: A systematic review of pharmacoepidemiological data". Journal of Evidence-based Medicine 14 (4): 278–280. December 2021. doi:10.1111/jebm.12456. PMID 34643998.
- ↑ "Therapy: Risks associated with chronic PPI use - signal or noise?". Nature Reviews. Gastroenterology & Hepatology 13 (5): 253–4. May 2016. doi:10.1038/nrgastro.2016.44. PMID 27006255. https://zenodo.org/record/895402.
- ↑ "Chapter 13. Proton-Potassium (H+/K+) ATPases: Properties and Roles in Health and Diseases". The Alkali Metal Ions: Their Role in Life. Metal Ions in Life Sciences. 16. Springer. 2016. pp. 459–483. doi:10.1007/978-3-319-21756-7_13.
- ↑ Fellenius, Erik; Berglindh, Thomas; Sachs, George; Olbe, Lars; Elander, Berit; Sjöstrand, Sven-Erik; Wallmark, Björn (March 1981). "Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+) ATPase". Nature 290 (5802): 159–161. doi:10.1038/290159a0. ISSN 0028-0836. PMID 6259537. Bibcode: 1981Natur.290..159F. http://dx.doi.org/10.1038/290159a0.
- ↑ Shin, Jai Moo; Sachs, George (November 2002). "Restoration of acid secretion following treatment with proton pump inhibitors". Gastroenterology 123 (5): 1588–1597. doi:10.1053/gast.2002.36593. ISSN 0016-5085. PMID 12404233.
- ↑ "Optimizing proton pump inhibitors in Helicobacter pylori treatment: Old and new tricks to improve effectiveness". World J Gastroenterol 25 (34): 5097–5104. September 2019. doi:10.3748/wjg.v25.i34.5097. PMID 31558859.
- ↑ "Proton pump inhibitors: better acid suppression when taken before a meal than without a meal". Alimentary Pharmacology & Therapeutics 14 (10): 1267–72. October 2000. doi:10.1046/j.1365-2036.2000.00829.x. PMID 11012470.
- ↑ AstraZeneca Pty Ltd. Nexium (Australian approved prescribing information). North Ryde: AstraZeneca; 2005.
- ↑ Wyeth Australia Pty Ltd. Zoton (Australian approved prescribing information). Baulkham Hills: Wyeth; 2004.
- ↑ 77.0 77.1 Shin, Jai Moo; Sachs, George (December 2008). "Pharmacology of proton pump inhibitors" (in en). Current Gastroenterology Reports 10 (6): 528–534. doi:10.1007/s11894-008-0098-4. ISSN 1522-8037. PMID 19006606.
- ↑ "The gastric HK-ATPase: structure, function, and inhibition". Pflügers Archiv 457 (3): 609–22. January 2009. doi:10.1007/s00424-008-0495-4. PMID 18536934.
- ↑ "Two of a kind". Chemistry in Britain 38 (5): 42–5. 2002.
- ↑ 80.0 80.1 "Lansoprazole, Dexlansoprazole". Clinical and Research Information on Drug-induced Liver Injury. National Institutes of Health (NIH). Retrieved May 8, 2018.
- ↑ 81.0 81.1 "Omeprazole and Esomeprazole". Clinical and Research Information on Drug-induced Liver Injury. National Institutes of Health (NIH). Retrieved May 8, 2018.
- ↑ "Pantoprazole". Clinical and Research Information on Drug-induced Liver Injury. National Institutes of Health (NIH). Retrieved May 8, 2018.
- ↑ "Rabeprazole". Clinical and Research Information on Drug-induced Liver Injury. National Institutes of Health (NIH). Retrieved May 8, 2018.
- ↑ 84.0 84.1 Comparing Proton Pump Inhibitors. PubMed Clinical Q&A. National Center for Biotechnology Information (US). 1 October 2010. https://www.ncbi.nlm.nih.gov/books/NBK51075/.
- ↑ "Clinical trial: inhibitory effect of revaprazan on gastric acid secretion in healthy male subjects". Journal of Gastroenterology and Hepatology 25 (10): 1618–25. October 2010. doi:10.1111/j.1440-1746.2010.06408.x. PMID 20880169.
- ↑ "Proton Pump Inhibitors in Primary Care". Province of British Columbia. January 2015. https://www2.gov.bc.ca/assets/gov/health/practitioner-pro/provincial-academic-detailing-service/pad-2015-proton-pump-inhibitors-newsletter.pdf.
- ↑ "25 Years of Proton Pump Inhibitors: A Comprehensive Review". Gut and Liver 11 (1): 27–37. January 2017. doi:10.5009/gnl15502. PMID 27840364.
External links
| Classification |
|---|
 |


