Chemistry:Xanthan gum
| |
| Names | |
|---|---|
| Other names
E 415
| |
| Identifiers | |
| ChemSpider |
|
| EC Number |
|
| UNII | |
| Properties | |
| C35H49O29 (monomer) | |
| Molar mass | 933.748 g·mol−1 |
| Hazards | |
| Safety data sheet | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xanthan gum (/ˈzænθən/) is a polysaccharide with many industrial uses, including as a common food additive. It is an effective thickening agent and stabilizer that prevents ingredients from separating. It can be produced from simple sugars by fermentation and derives its name from the species of bacteria used, Xanthomonas campestris.
History
Xanthan gum was discovered by Allene Rosalind Jeanes and her research team at the United States Department of Agriculture, and brought into commercial production by CP Kelco under the trade name Kelzan in the early 1960s.[2][3] It was approved for use in foods in 1968 and is accepted as a safe food additive in the US, Canada, European countries, and many other countries, with E number E415, and CAS number 11138-66-2.
Xanthan gum derives its name from the species of bacteria used during the fermentation process, Xanthomonas campestris.[4]
Uses
Xanthan gum, 1%, can produce a significant increase in the viscosity of a liquid.[5]
In foods, xanthan gum is common in salad dressings and sauces. It helps to prevent oil separation by stabilizing the emulsion, although it is not an emulsifier. Xanthan gum also helps suspend solid particles, such as spices. Xanthan gum helps create the desired texture in many ice creams. Toothpaste often contains xanthan gum as a binder to keep the product uniform. Xanthan gum also helps thicken commercial egg substitutes made from egg whites, to replace the fat and emulsifiers found in yolks. It is also a preferred method of thickening liquids for those with swallowing disorders, since it does not change the color or flavor of foods or beverages at typical use levels.[6] In gluten-free baking, xanthan gum is used to give the dough or batter the stickiness that would otherwise be achieved with gluten. In most foods, it is used at concentrations of 0.5% or less. Xanthan gum is used in a wide range of food products, such as sauces, dressings, meat and poultry products, bakery products, confectionery products, beverages, dairy products, and others.
In the oil industry, xanthan gum is used in large quantities to thicken drilling mud.[7] These fluids carry the solids cut by the drilling bit to the surface. Xanthan gum provides improved "low end" rheology. When circulation stops, the solids remain suspended in the drilling fluid. The widespread use of horizontal drilling and the demand for good control of drilled solids has led to its expanded use. It has been added to concrete poured underwater, to increase its viscosity and prevent washout.
In cosmetics, xanthan gum is used to prepare water gels.[8] It is also used in oil-in-water emulsions to enhance droplet coalescence.[9] Xanthan gum is under preliminary research for its potential uses in tissue engineering to construct hydrogels and scaffolds supporting three-dimensional tissue formation.[8] Furthermore, thiolated xanthan gum (see thiomers) has shown potential for drug delivery,[10][11] since by the covalent attachment of thiol groups to this polysaccharide high mucoadhesive and permeation enhancing properties can be introduced.[12]
Shear thinning
The viscosity of xanthan gum solutions decreases with higher shear rates. This is called shear thinning or pseudoplasticity. This means that a product subjected to shear, whether from mixing, shaking or chewing will thin. When the shear forces are removed, the food will thicken again. In salad dressing, the addition of xanthan gum makes it thick enough at rest in the bottle to keep the mixture fairly homogeneous, but the shear forces generated by shaking and pouring thins it, so it can be easily poured. When it exits the bottle, the shear forces are removed and it thickens again, so it clings to the salad.
Concentrations used
The greater the concentration of xanthan gum in a liquid, the thicker the liquid will become. An emulsion can be formed with as little as 0.1% (by weight). Increasing the concentration of gum gives a thicker, more stable emulsion up to 1% xanthan gum. A teaspoon of xanthan gum weighs about 2.5 grams and brings one cup (250 ml) of water to a 1% concentration.[6][13]
To make a foam, 0.2–0.8% xanthan gum is typically used. Larger amounts result in larger bubbles and denser foam. Egg white powder (0.2–2.0%) with 0.1–0.4% xanthan gum yields bubbles similar to soap bubbles.
Health
Xanthan gum may have some health benefits. It has slowed tumor growth in mice with skin cancer,[14] stabilized blood sugar,[15] lowered cholesterol [16] and improved the symptoms of dysphagia.[17] Xanthan gum may also act as a laxative.[18]
Safety
According to a 2017 safety review by a scientific panel of the European Food Safety Authority (EFSA), xanthan gum (European food additive number E 415) is extensively digested during intestinal fermentation, and causes no adverse effects, even at high intake amounts.[19] The EFSA panel found no concern about genotoxicity from long-term consumption.[19] The EFSA concluded that there is no safety concern for the general population when xanthan gum is consumed as a food additive.[19]
Processing by microbiome
In 2022, scientists found that a microbe from the family Ruminococcaceae, present in human stool samples, was capable of degrading xanthan gum, and appeared to be from the microbiome of people in industrialized countries.[20]
Preparation
Xanthan gum is produced by the fermentation of glucose and sucrose.[citation needed] The medium is well-aerated and stirred, and the xanthan polymer is produced extracellularly into the medium. After one to four days, the polymer is precipitated from the medium by the addition of isopropyl alcohol, and the precipitate is dried and milled to give a powder that is readily soluble in water or brine.[19]
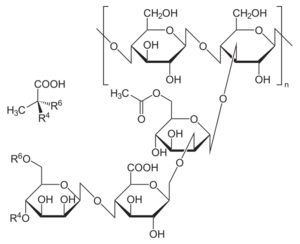
It is composed of pentasaccharide repeat units, comprising glucose, mannose, and glucuronic acid in the molar ratio 2:2:1.[19][21]
A strain of X. campestris that will grow on lactose has been developed – which allows it to be used to process whey, a waste product of cheese production. This can produce 30 g/L of xanthan gum for every 40 g/L of whey powder. Whey-derived xanthan gum is commonly used in many commercial products, such as shampoos and salad dressings.[22]
Detail of the biosynthesis
Synthesis originates from glucose as substrate for synthesis of the sugar nucleotides precursors UDP-glucose, UDP-glucuronate, and GDP-mannose that are required for building the pentasaccharide repeat unit.[19] This links the synthesis of xanthan to carbohydrate metabolism. The repeat units are built up at undecaprenylphosphate lipid carriers that are anchored in the cytoplasmic membrane.[citation needed]
Specific glycosyltransferases sequentially transfer the sugar moieties of the nucleotide sugar xanthan precursors to the lipid carriers. Acetyl and pyruvyl residues are added as non-carbohydrate decorations. Mature repeat units are polymerized and exported in a way resembling the Wzy-dependent polysaccharide synthesis mechanism of Enterobacteriaceae. Products of the gum gene cluster drive synthesis, polymerization, and export of the repeat unit.[23]
References
- ↑ "Sicherheitsdatenblatt des Herstellers Carl-Roth". http://www.carl-roth.de/jsp/de-de/sdpdf/3557.PDF.
- ↑ Whistler, Roy, L, and BeMiller, James N., eds Industrial Gums: Polysaccharides and their Derivatives Academic Press (1973) ISBN:0-12-746252-X.
- ↑ "KELZAN Xanthan Gum - CP Kelco". CP Kelco. Feb 18, 2019. https://www.cpkelco.com/markets-served/household-products/products/kelzan-xanthan-gum/. "CP Kelco offers a range of biopolymers to thicken, suspend and stabilize emulsions and other water-based systems. The KELZAN xanthan gum line of industrial products can be used to modify the texture of industrial products and to stabilize household cleaners, fabric care products, suspensions, oil-in-water emulsions and foams against separation."
- ↑ Barrére, G.C., C.E. Barber, and M.J. Daniels (1986) Intl. J. Biological Macromolecules, 8(6):372-374
- ↑ Davidson, Robert L. (1980). Handbook of Water-soluble Gums and Resins. McGraw Hill. ISBN 978-0-07-015471-1.
- ↑ 6.0 6.1 cuisine, m. (2014). Xanthan Gum. Retrieved from modernist cuisine: "Xanthan Gum". 2012-11-27. http://www.modernistcookingmadeeasy.com/info/modernist-ingredients/more/xanthan-gum.
- ↑ "Oilfield Glossary - xanthan gum". Schlumberger. http://www.glossary.oilfield.slb.com/Terms/x/xanthan_gum.aspx.
- ↑ 8.0 8.1 Kumar, A.; Rao, K. M.; Han, S. S. (2018). "Application of xanthan gum as polysaccharide in tissue engineering: A review". Carbohydrate Polymers 180: 128–144. doi:10.1016/j.carbpol.2017.10.009. PMID 29103488.
- ↑ Ye, Aiqian; Hemar, Yacine; Singh, Harjinder (2004-08-25). "Influence of polysaccharides on the rate of coalescence in oil-in-water emulsions formed with highly hydrolyzed whey proteins". Journal of Agricultural and Food Chemistry 52 (17): 5491–5498. doi:10.1021/jf030762o. ISSN 0021-8561. PMID 15315390.
- ↑ Bhatia, M; Ahuja, M; Mehta, H (2015). "Thiol derivatization of Xanthan gum and its evaluation as a mucoadhesive polymer". Carbohydr Polym 131: 119–124. doi:10.1016/j.carbpol.2015.05.049. PMID 26256167.
- ↑ Alhakamy, NA; Naveen, NR; Gorityala, S; Kurakula, M; Hosny, KM; Safhi, AY (2022). "Development of Novel S-Protective Thiolated-Based Mucoadhesive Tablets for Repaglinide: Pharmacokinetic Study". Polymers 14 (17): 3529. doi:10.3390/polym14173529. PMID 36080604.
- ↑ Leichner, C; Jelkmann, M; Bernkop-Schnürch, A (2019). "Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature". Adv Drug Deliv Rev 151-152: 191–221. doi:10.1016/j.addr.2019.04.007. PMID 31028759.
- ↑ Tests and measurements of xanthan gum "Xanthan gum: Get past the weird and it's magical". http://feedme.typepad.com/my_weblog/2016/01/xanthan-gum-magical-food-thickener.html.
- ↑ Takeuchi, Ario (2009). "Oral administration of xanthan gum enhances antitumor activity through Toll-like receptor 4". International Immunopharmacology 9 (13–14): 1562–1567. doi:10.1016/j.intimp.2009.09.012. PMID 19788935. https://pubmed.ncbi.nlm.nih.gov/19788935/.
- ↑ Fuwa, Masako (2016). "Effect of Xanthan Gum on Blood Sugar Level after Cooked Rice Consumption". Food Science and Technology Research 22: 117–126. doi:10.3136/fstr.22.117. https://www.jstage.jst.go.jp/article/fstr/22/1/22_117/_article.
- ↑ Eastwood, MA (1987). "The dietary effects of xanthan gum in man". Food Additives and Contaminants 4 (1): 17–26. doi:10.1080/02652038709373610. PMID 3549377. https://pubmed.ncbi.nlm.nih.gov/3549377/.
- ↑ Rofes, L (2014). "The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia". Alimentary Pharmacology & Therapeutics 39 (10): 1169–1179. doi:10.1111/apt.12696. PMID 24628492.
- ↑ Chengquan, Tan (2017). "Effects of dietary fibers with high water-binding capacity and swelling capacity on gastrointestinal functions, food intake and body weight in male rats". Food & Nutrition Research 61 (1). doi:10.1080/16546628.2017.1308118. PMID 28469548.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 EFSA Panel on Food Additives and Nutrient Sources (14 July 2017). "Re-evaluation of xanthan gum (E 415) as a food additive". EFSA Journal (European Food Safety Authority) 15 (2): e04909. doi:10.2903/j.efsa.2017.4909. PMID 32625570.
- ↑ Ostrowski, Matthew P.; La Rosa, Sabina Leanti; Kunath, Benoit J. et al. (April 2022). "Mechanistic insights into consumption of the food additive xanthan gum by the human gut microbiota". Nature Microbiology 7 (4): 556–569. doi:10.1038/s41564-022-01093-0. PMID 35365790.
- ↑ Garcı́a-Ochoa, F; Santos, V.E; Casas, J.A; Gómez, E (2000). "Xanthan gum: production, recovery, and properties". Biotechnology Advances 18 (7): 549–579. doi:10.1016/S0734-9750(00)00050-1. ISSN 0734-9750. PMID 14538095.
- ↑ Tortora, G.J., Funke, B.R., & Case, C.L. (2010). Microbiology: An Introduction, 10th edition. San Francisco: Benjamin Cummings. Pg. 801.
- ↑ Becker and Vorholter (2009). "Xanthan Biosynthesis by Xanthomonas Bacteria: An Overview of the Current Biochemical and Genomic Data". Microbial Production of Biopolymers and Polymer Precursors. Caister Academic Press. ISBN 978-1-904455-36-3.
 |

