Chemistry:Barium manganate
| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| BaMnO4 | |
| Molar mass | 256.26 g/mol |
| Appearance | light blue to dark blue and black powder |
| Density | 4.85 g/cm3 |
| insoluble[1] | |
| Hazards | |
| Main hazards | GHS03, GHS07: oxidizing, skin and eye irritant |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
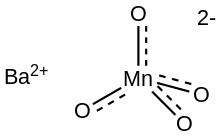
Barium manganate is an inorganic compound with the formula BaMnO4. It is used as an oxidant in organic chemistry.[2] It belongs to a class of compounds known as manganates in which the manganese resides in a +6 oxidation state. Manganate should not be confused with permanganate which contains manganese(VII). Barium manganate is a powerful oxidant, popular in organic synthesis and can be used in a wide variety of oxidation reactions.
Properties
The manganate(VI) ion is a d1 ion and is tetrahedral with bond angles of approximately 109.5°. The Mn−O bond lengths in BaMnO4 and K2MnO4 are identical at 1.66 Å. In comparison, the Mn-O bond length in MnO2−
4 is longer than in MnO4− of 1.56 Å and shorter than the Mn−O bond found in MnO2, 1.89 Å.[3][4] Barium manganate is isomorphous with BaCrO4 and BaSO4. Barium manganate can appear as a dark blue or green to black crystals.[5] Barium manganate is indefinitely stable, active and can be stored for months in dry conditions.[5]
Preparation
Barium manganate can be prepared from potassium manganate and barium chloride by salt metathesis to give insoluble barium manganate:[6]
- BaCl
2 + K
2MnO
4 → 2 KCl + BaMnO
4↓
Uses in organic synthesis
Barium manganate oxidizes a number of functional groups efficiently and selectively: alcohols to carbonyls, diols to lactones, thiols to disulfides, aromatic amines to azo-compounds, hydroquinone to p-benzoquinone, benzylamine to benzaldehyde, hydrazones to diazo compounds,
etc.[7] It does not oxidize saturated hydrocarbons, alkenes, unsaturated ketones, and tertiary amines. Barium manganate is a common substitute for MnO2. It is easier to prepare, reacts more efficiently, and the substrate:oxidant ratios are closer to theory.
Another use for barium manganate was as a reagent in the synthesis of the inorganic pigment manganese blue, which is no longer produced on an industrial scale.
References
- ↑ Olsen, J. C. (1900). Permanganic Acid by Electrolysys. Easton, PA: The Chemical Publishing Company. https://books.google.com/books?id=EJNPAAAAYAAJ.
- ↑ Garry Procter, Steven V. Ley, Grant H. Castle, "Barium Manganate" Encyclopedia of Reagents for Organic Synthesis 2001. doi:10.1002/047084289X.rb003
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford:Pergamon Press. Vol. 15, "Manganese Compounds". ISBN:0-08-022057-6.
- ↑ Jellinek, F. J. Inorg. Nucl. Chem. 1960. 13, 329-331. {{doi: 10.1016/0022-1902(60)80316-8}}
- ↑ 5.0 5.1 Firouzabadi, H.; Mostafavipoor,Z. (1983), "Barium Manganate. A Versatile Oxidant in Organic Synthesis", Bull. Chem. Soc. Jpn. 56 (3): p914-917. {{doi: 10.1246/bcsj.56.914}}.
- ↑ Carrington, A.; Symons, M. C. R. "Structure and reactivity of the oxy-anions of transition metals. Part I. The managese oxy-anions", J. Chem. Soc. 1956, p3373-3380. doi:10.1039/JR9560003373.
- ↑ Procter.G.; Ley, S. V.; Castle, G.H. (2004), "Barium Manganate", in Paquette,L., Encyclopedia of Reagents for Organic Synthesis, New York:Wiley, {{doi:10.1002/047084289X}}.
 |

