Chemistry:Chlorothiazide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Diuril, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682341 |
| Pregnancy category |
|
| Routes of administration | By mouth, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | low |
| Metabolism | Nil |
| Elimination half-life | 45 to 120 minutes |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
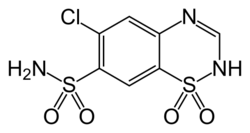
| Formula | C7H6ClN3O4S2 |
| Molar mass | 295.71 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
| Names | |
|---|---|
| Other names
6-Chloro-2H-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Melting point | 342.5–343 °C (648.5–649.4 °F; 615.6–616.1 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chlorothiazide, sold under the brand name Diuril among others, is an organic compound used as a diuretic and as an antihypertensive.[1][2]
It is used both within the hospital setting or for personal use to manage excess fluid associated with congestive heart failure. Most often taken in pill form, it is usually taken orally once or twice a day. In the ICU setting, chlorothiazide is given to diurese a patient in addition to furosemide (Lasix). Working in a separate mechanism from furosemide and absorbed enterically as a reconstituted suspension administered through a nasogastric tube (NG tube), the two drugs potentiate one another.
It was patented in 1956 and approved for medical use in 1958.[3] It is on the World Health Organization's List of Essential Medicines.[4]
Indications
- Large amount of excess fluid including:
- Heart failure
- Peripheral edema
- Hypertension
Contraindications
Side effects
- Nausea / Vomiting
- Headache
- Dizziness
- Excess urine production
- Dehydration
- Hypoelectrolytemia (esp. hypokalemia / hypomagnesia)
History
The research team of Merck Sharp and Dohme Research Laboratories of Beyer, Sprague, Baer, and Novello created a new series of medications, the thiazide diuretics, which includes chlorothiazide. They won an Albert Lasker Special Award in 1975 for this work.[6]
The structure has been determined by X-ray crystallography.[7]
See also
References
- ↑ "Thiazide diuretics: 50 years and beyond.". Current Hypertension Reviews 4 (4): 256–65. November 2008. doi:10.2174/157340208786241264.
- ↑ "Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics". Expert Review of Cardiovascular Therapy 8 (6): 793–802. June 2010. doi:10.1586/erc.10.27. PMID 20528637.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 456. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA456.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ Jump up to: 5.0 5.1 "Diuril (Chlorothiazide): Side Effects, Interactions, Warning, Dosage & Uses" (in en). https://www.rxlist.com/diuril-drug.htm.
- ↑ "Historical Awards - The Lasker Foundation". The Lasker Foundation. http://www.laskerfoundation.org/awards/formaward.htm.
- ↑ "Experimental and Predicted Crystal Energy Landscapes of Chlorothiazide". Crystal Growth & Design 11 (2): 405–413. 2011. doi:10.1021/cg1010049.
 |

