Chemistry:Laquinimod
| |
| Names | |
|---|---|
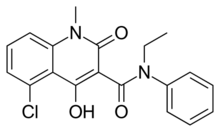
| Preferred IUPAC name
5-Chloro-N-ethyl-4-hydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-3-carboxamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H17ClN2O3 | |
| Molar mass | 356.803 g/mol |
| Pharmacology | |
| 1=ATC code }} | N07XX10 (WHO) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Laquinimod is an experimental immunomodulator developed by Active Biotech and Teva. It is being investigated as an oral treatment for multiple sclerosis (MS) and Huntington's disease.
Laquinimod is the successor of Active Biotech's failed experimental immunomodulator linomide.[1]
The compound has been investigated in two Phase II trials using successive magnetic resonance scans (MRI). Laquinimod seems to be able to reduce the MS disease activity on MRI.[2][3][4] However, the response to a given dose was discrepant between both studies.[5]
Phase III studies for MS started in December 2007.[6] In 2011, Teva announced its clinical trials involving laquinimod had failed, being unable to significantly reduce relapses in MS among patients beyond a placebo.[7] However, the final results of above-mentioned phase III trial proved oral laquinimod administered once daily slowed the progression of disability and reduced the rate of relapse in patients with relapsing–remitting multiple sclerosis.[8] [clarification needed]
On May 7, 2013 laquinimod was approved by the Russian Ministry of Health (the FDA analog) as a treatment for relapsing-remitting multiple sclerosis (RRMS) under the brand name Nerventra.[9]
See also
References
- ↑ Tan IL; Lycklama à Nijeholt GJ; Polman CH et al. (April 2000). "Linomide in the treatment of multiple sclerosis: MRI results from prematurely terminated phase-III trials". Mult Scler 6 (2): 99–104. doi:10.1191/135245800678827626. PMID 10773855.
- ↑ Comi G; Pulizzi A; Rovaris M et al. (June 2008). "Effect of laquinimod on MRI-monitored disease activity in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study". Lancet 371 (9630): 2085–2092. doi:10.1016/S0140-6736(08)60918-6. PMID 18572078.
- ↑ Polman C; Barkhof F; Sandberg-Wollheim M et al. (March 2005). "Treatment with laquinimod reduces development of active MRI lesions in relapsing MS". Neurology 64 (6): 987–91. doi:10.1212/01.WNL.0000154520.48391.69. PMID 15781813.
- ↑ He, Dian; Han, Kai; Gao, Xiangdong; Dong, Shuai; Chu, Lan; Feng, ZhanHui; Wu, Shan (2013-08-06). Chu, Lan. ed. "Laquinimod for multiple sclerosis". The Cochrane Database of Systematic Reviews (8): CD010475. doi:10.1002/14651858.CD010475.pub2. ISSN 1469-493X. PMID 23922214.
- ↑ Keegan BM, Weinshenker BG (June 2008). "Laquinimod, a new oral drug for multiple sclerosis". Lancet 371 (9630): 2059–2060. doi:10.1016/S0140-6736(08)60894-6. PMID 18572062.
- ↑ Clinical trial number NCT00509145 for "Safety and Efficacy of Orally Administered Laquinimod Versus Placebo for Treatment of Relapsing Remitting Multiple Sclerosis (RRMS) (ALLEGRO)" at ClinicalTrials.gov
- ↑ Kresege, Naomi (1 August 2011). "Teva's Copaxone Successor Fails in Latest Clinical Trial". Bloomberg. https://www.bloomberg.com/news/2011-08-01/teva-s-oral-multiple-sclerosis-drug-fails-to-meet-goal-of-clinical-trial.html. "Teva Pharmaceutical Industries Ltd. (TEVA)’s experimental multiple sclerosis pill failed to reduce relapses more than placebo in a clinical trial, dealing a blow to the company’s effort to find a successor to an older drug."
- ↑ Comi, G.; Jeffery, D.; Kappos, L.; Montalban, X.; Boyko, A.; Rocca, M. A.; Filippi, M.; Allegro Study, G. (2012). "Placebo-Controlled Trial of Oral Laquinimod for Multiple Sclerosis". New England Journal of Medicine 366 (11): 1000–1009. doi:10.1056/NEJMoa1104318. PMID 22417253.
- ↑ "Nerventra (laquinimod) Capsules 0,6 mg. Registration certificate" (in ru). http://grls.rosminzdrav.ru/Grls_View_v2.aspx?idReg=64645&t=.
External links
 |

