Chemistry:Potassium hexanitritocobaltate(III)
| |

| |
| Names | |
|---|---|
| IUPAC name
Potassium hexanitritocobaltate(III)
| |
| Other names
Potassium cobaltinitrite
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| K3[Co(NO2)6] (anhydrous) K3[Co(NO2)6]·1.5H2O (sesquihydrate) | |
| Molar mass | 452.26 g/mol (anhydrous) 479.284 g/mol (sesquihydrate) |
| Appearance | yellow cubic crystals (sesquihydrate) |
| Density | 2.6 g/cm3 (sesquihydrate) |
| slightly soluble in water (sesquihydrate) | |
| Solubility | reacts with acids, insoluble in ethanol (sesquihydrate)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
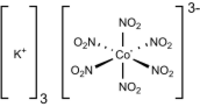
Potassium hexanitritocobaltate(III) is a salt with the formula K3[Co(NO2)6]. It is a yellow solid that is poorly soluble in water. The compound finds some use as a yellow pigment under the name Indian Yellow.
The salt features potassium cations and an trianionic coordination complex. In the anion, cobalt is bound by six nitrito ligands, the overall complex having octahedral molecular geometry. The oxidation state of cobalt is 3+. Its low-spin d6 configuration confers kinetic stability and diamagnetism. The compound is prepared by combining cobalt(II) and nitrite salts in the presence of oxygen. The corresponding sodium cobaltinitrite is significantly more soluble in water.[2]
The compound was first described in 1848 by Nikolaus Wolfgang Fischer in Breslau,[3] and it is used as a yellow pigment called Aureolin.[4][5]
See also
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–54. ISBN 0-8493-0594-2.
- ↑ O. Glemser (1963). "Sodium Hexanitritocobaltate (III)". in G. Brauer. Handbook of Preparative Inorganic Chemistry, 2nd Ed.. 1. NY, NY: Academic Press. pp. 1541.
- ↑ Fischer, N. W. (1848). "Ueber die salpetrichtsauren Salze". Annalen der Physik und Chemie 150 (5): 115–125. doi:10.1002/andp.18491500512. Bibcode: 1848AnP...150..115F. https://zenodo.org/record/1423620.
- ↑ Gates, G. (1995). "A Note on the Artists' Pigment Aureolin". Studies in Conservation 40 (3): 201–206. doi:10.2307/1506479.
- ↑ Gettens, Rutherford John; Stout, George Leslie (1966). Painting materials: A short encyclopaedia. Courier Corporation. pp. 109–110. ISBN 978-0-486-21597-6. https://books.google.com/books?id=bdQVgKWl3f4C&pg=PA109.
 |

