Chemistry:Lithium bis(trimethylsilyl)amide
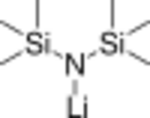 Monomer (does not exist)
| |
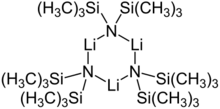 Cyclic trimer
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Lithium 1,1,1-trimethyl-N-(trimethylsilyl)silanaminide | |
| Other names
Lithium hexamethyldisilazide
Hexamethyldisilazane lithium salt | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| LiN(Si(CH 3) 3) 2 | |
| Molar mass | 167.33 g·mol−1 |
| Appearance | White solid |
| Density | 0.86 g/cm3 at 25 °C |
| Melting point | 71 to 72 °C (160 to 162 °F; 344 to 345 K) |
| Boiling point | 80 to 84 °C (176 to 183 °F; 353 to 357 K) (0.001 mm Hg) |
| decomposes | |
| Solubility | Most aprotic solvents THF, hexane, toluene |
| Acidity (pKa) | 26 |
| Hazards | |
| Main hazards | flammable, corrosive |
| Related compounds | |
Related compounds
|
Sodium bis(trimethylsilyl)amide Potassium bis(trimethylsilyl)amide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(Si(CH
3)
3)
2. It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.
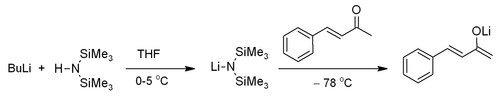
Preparation
LiHMDS is commercially available, but it can also be prepared by the deprotonation of bis(trimethylsilyl)amine with n-butyllithium.[1] This reaction can be performed in situ.[2]
- HN(Si(CH
3)
3)
2 + C
4H
9Li → LiN(Si(CH
3)
3)
2 + C
4H
10
Once formed, the compound can be purified by sublimation or distillation.
Reactions and applications
As a base
LiHMDS is often used in organic chemistry as a strong non-nucleophilic base.[3] Its conjugate acid has a pKa of ~26,[4] making it is less basic than other lithium bases, such as LDA (pKa of conjugate acid ~36). It is relatively more sterically hindered and hence less nucleophilic than other lithium bases. It can be used to form various organolithium compounds, including acetylides[3] or lithium enolates.[2]
where Me = CH
3. As such, it finds use in a range of coupling reactions, particularly carbon-carbon bond forming reactions such as the Fráter–Seebach alkylation and mixed Claisen condensations.
An alternative synthesis of tetrasulfur tetranitride entails the use of S(N(Si(CH
3)
3)
2)
2 as a precursor with pre-formed S–N bonds. S(N(Si(CH
3)
3)
2)
2 is prepared by the reaction of lithium bis(trimethylsilyl)amide and sulfur dichloride (SCl
2).
- 2 LiN(Si(CH
3)
3)
2 + SCl
2 → S(N(Si(CH
3)
3)
2)
2 + 2 LiCl
The S(N(Si(CH
3)
3)
2)
2 reacts with the combination of SCl
2 and sulfuryl chloride (SO
2Cl
2) to form S
4N
4, trimethylsilyl chloride, and sulfur dioxide:[5]
- 2 S(N(Si(CH
3)
3)
2)
2 + 2 SCl
2 + 2 SO
2Cl
2 → S
4N
4 + 8 (CH
3)
3SiCl + 2 SO
2
As a ligand
Li(HMDS) can react with a wide range of metal halides, by a salt metathesis reaction, to give metal bis(trimethylsilyl)amides.
- MX
n + n Li(HMDS) → M(HMDS)
n + n LiX
where X = Cl, Br, I and sometimes F
Metal bis(trimethylsilyl)amide complexes are lipophilic due to the ligand and hence are soluble in a range of nonpolar organic solvents, this often makes them more reactive than the corresponding metal halides, which can be difficult to solubilise. The steric bulk of the ligands causes their complexes to be discrete and monomeric; further increasing their reactivity. Having a built-in base, these compounds conveniently react with protic ligand precursors to give other metal complexes and hence are important precursors to more complex coordination compounds.[6]
Niche uses
LiHMDS is volatile and has been discussed for use for atomic layer deposition of lithium compounds.[7]
Structure
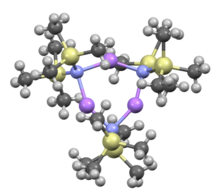
Like many organolithium reagents, lithium bis(trimethylsilyl)amide can form aggregates in solution. The extent of aggregation depends on the solvent. In coordinating solvents, such as ethers[8] and amines,[9] the monomer and dimer are prevalent. In the monomeric and dimeric state, one or two solvent molecules bind to lithium centers. With ammonia as donor base lithium bis(trimethylsilyl)amide forms a trisolvated monomer that is stabilized by intermolecular hydrogen bonds.[10][11] In noncoordinating solvents, such as aromatics or pentane, the complex oligomers predominate, including the trimer.[9] In the solid state structure is trimeric.[12]
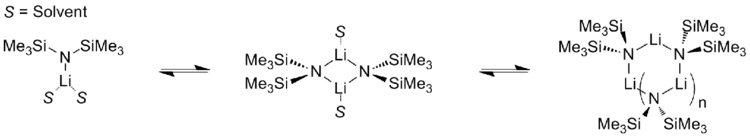
| ||||
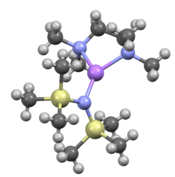 LiHMDS adduct with TMEDA |
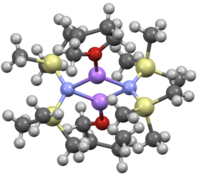 THF solvated dimer: [(LiHMDS) 2(THF) 2] |
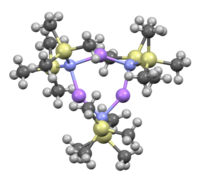 Trimer, solvent free: [(LiHMDS) 3] | ||
See also
References
- ↑ Amonoo-Neizer, E. H.; Shaw, R. A.; Skovlin, D. O.; Smith, B. C. (1966). "Lithium Bis(trimethylsilyl)amide and Tris(trimethylsilyl)amine". Inorganic Syntheses. 8. 19–22. doi:10.1002/9780470132395.ch6. ISBN 978-0-470-13239-5.
- ↑ 2.0 2.1 Danheiser, R. L.; Miller, R. F.; Brisbois, R. G. (1990). "Detrifluoroacetylative Diazo Group Transfer: (E)-1-Diazo-4-phenyl-3-buten-2-one". Organic Syntheses 73: 134. http://www.orgsyn.org/demo.aspx?prep=CV9P0197.; Collective Volume, 9, pp. 197
- ↑ 3.0 3.1 Wu, George; Huang, Mingsheng (July 2006). "Organolithium Reagents in Pharmaceutical Asymmetric Processes". Chemical Reviews 106 (7): 2596–2616. doi:10.1021/cr040694k. PMID 16836294.
- ↑ Fraser, Robert R.; Mansour, Tarek S.; Savard, Sylvain (August 1985). "Acidity measurements on pyridines in tetrahydrofuran using lithiated silylamines". The Journal of Organic Chemistry 50 (17): 3232–3234. doi:10.1021/jo00217a050.
- ↑ Maaninen, A.; Shvari, J.; Laitinen, R. S.; Chivers, T (2002). "Compounds of General Interest". in Coucouvanis, Dimitri. Inorganic Syntheses. 33. New York: John Wiley & Sons, Inc.. pp. 196–199. doi:10.1002/0471224502.ch4. ISBN 9780471208259.
- ↑ Michael Lappert, Andrey Protchenko, Philip Power, Alexandra Seeber (2009). Metal Amide Chemistry. Weinheim: Wiley-VCH. doi:10.1002/9780470740385. ISBN 978-0-470-72184-1.
- ↑ Hämäläinen, Jani; Holopainen, Jani; Munnik, Frans; Hatanpää, Timo; Heikkilä, Mikko; Ritala, Mikko; Leskelä, Markku (2012). "Lithium Phosphate Thin Films Grown by Atomic Layer Deposition". Journal of the Electrochemical Society 159 (3): A259–A263. doi:10.1149/2.052203jes.
- ↑ Lucht, Brett L.; Collum, David B. (1995). "Ethereal Solvation of Lithium Hexamethyldisilazide: Unexpected Relationships of Solvation Number, Solvation Energy, and Aggregation State". Journal of the American Chemical Society 117 (39): 9863–9874. doi:10.1021/ja00144a012.
- ↑ 9.0 9.1 Lucht, Brett L.; Collum, David B. (1996). "Lithium Ion Solvation: Amine and Unsaturated Hydrocarbon Solvates of Lithium Hexamethyldisilazide (LiHMDS)". Journal of the American Chemical Society 118 (9): 2217–2225. doi:10.1021/ja953029p.
- ↑ Neufeld, R.; Michel, R.; Herbst-Irmer, R.; Schöne, R.; Stalke, D. (2016). "Introducing a Hydrogen-Bond Donor into a Weakly Nucleophilic Brønsted Base: Alkali Metal Hexamethyldisilazides (MHMDS, M = Li, Na, K, Rb and Cs) with Ammonia". Chem. Eur. J. 22 (35): 12340–12346. doi:10.1002/chem.201600833. PMID 27457218.
- ↑ Neufeld, R.: DOSY External Calibration Curve Molecular Weight Determination as a Valuable Methodology in Characterizing Reactive Intermediates in Solution. In: eDiss, Georg-August-Universität Göttingen. 2016.
- ↑ Rogers, Robin D.; Atwood, Jerry L.; Grüning, Rainer (1978). "The crystal structure of N-lithiohexamethyldisilazane, [LiN(SiMe3)2]3". J. Organomet. Chem. 157 (2): 229–237. doi:10.1016/S0022-328X(00)92291-5.
 |