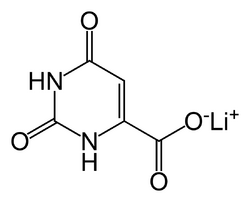
Chemistry:Lithium orotate
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C5H3LiN2O4 |
| Molar mass | 162.03 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Lithium orotate (C5H3LiN2O4) is a salt of orotic acid and lithium. It is available as the monohydrate, LiC5H3N2O4·H2O.[1] In this compound, lithium is non-covalently bound to an orotate ion, rather than to a carbonate or other ion, and like other salts, dissociates in solution to produce free lithium ions. It is marketed as a dietary supplement, though it has been researched minimally between 1973–1986 to treat certain medical conditions, such as alcoholism[2] and Alzheimer's disease.
While lithium orotate is capable of providing lithium to the body,[3] like lithium carbonate and other lithium salts, there are no systematic reviews supporting the efficacy of lithium orotate and it is not approved by the U.S. Food and Drug Administration (FDA) for the treatment of any medical condition.
Effectiveness
In 1973, Hans Nieper reported that lithium orotate contained 3.83 mg of elemental lithium per 100 mg and lithium carbonate contained 18.8 mg of elemental lithium per 100 mg.[4] Nieper went on to claim that lithium did not dissolve from the orotate carrier until it passed through the blood–brain barrier; however, a 1976 study documented that lithium concentrations within the brains of rats were not statistically different between equivalent dosages of lithium from lithium orotate, lithium carbonate, or lithium chloride.[5] However, another study in 1978 study showed that eight hours after intraperitoneal injections brain lithium concentrations of rats were significantly greater after lithium orotate than after lithium carbonate. While little serum lithium remained at 24 h after injection of 2·0 m equiv kg−1 lithium carbonate, two‐thirds of the 2 h serum lithium concentration was present 24 h after lithium orotate. Furthermore, the 24 h brain concentration of lithium after lithium orotate was approximately three times greater than that after lithium carbonate. These data suggest the possibility that lower doses of lithium orotate than lithium carbonate may achieve therapeutic brain lithium concentrations and relatively stable serum concentrations.[6] A year later, Smith and Schou repeated the experiment at a higher dose (2 mM Li+) and found that the higher concentrations in the brain could be possibly accounted for by decreased renal function in rats treated with lithium orotate.[7][8] The proponents of lithium orotate have since criticized the results by citing the fact that the dose of lithium orotate used in the study was in the toxic range. In 2022, Pacholko redid the experiment and showed lithium orotate to have a safer kidney profile than lithium carbonate, it also showed that both had an increased TSH only in females, but the increase was lower in the orotate group.[9]
The pharmacokinetics of lithium orotate in human brains is poorly documented, and there is no known mechanism by which orotate ions could alter the pharmacokinetics of dissociated lithium ions. Major medical research has not been conducted on lithium orotate since the 1980s.[citation needed] As previously stated, lithium intake appears to be effective even at low doses, and this may account for lithium orotate's claimed effectiveness.[10][11][12]
Safety
Preliminary studies seem to indicate that lithium orotate is safe if taken at lower dosages; a 6 month alcoholism cessation study led to only minor adverse effects in 8 out of 42 patients.[2] However, the lack of safety studies and the OTC status raises concerns.[8] Attention to it was specially raised in medical literature after a case report of an 18 year old woman with mild, acute lithium toxicity after taking an overdose (2.16 grams) of a lithium orotate supplement. She was discharged after treatment.[13][14][15] Lithium blood levels 90 minutes after ingestion reached 0.31 mEq/L, and an hour later after treatment, 0.40 mEq/L, levels below the serum toxicity level of 1.5 milliequivalents per liter (mEq/L).[14][16]
Orotic acid can be mutagenic in very high doses of 50 mg/kg in mammalian somatic cells.[17] It is also mutagenic for bacteria and yeast.[18] Although lithium orotate was not shown to be genotoxic,[19] short and long term studies must be performed to guarantee public safety.[8]
See also
References
- ↑ "Orotate complexes. Synthesis and crystal structure of lithium orotate( - I) monohydrate and magnesium bis[ orotate( - I)] octahydrate". Chemische Berichte 123 (12): 2267–2271. 1990. doi:10.1002/cber.19901231207.
- ↑ 2.0 2.1 "Lithium orotate in the treatment of alcoholism and related conditions". Alcohol 3 (2): 97–100. 1986. doi:10.1016/0741-8329(86)90018-2. PMID 3718672.
- ↑ "Lithium orotate: A superior option for lithium therapy?". Brain and Behavior 11 (4): e2262. 2021. doi:10.1002/brb3.2262. PMID 34196467.
- ↑ "The clinical applications of lithium orotate. A two years study". Agressologie 14 (6): 407–11. 1973. PMID 4607169. https://books.google.com/books?id=iuNKAAAAYAAJ.
- ↑ "Lithium orotate, carbonate and chloride: pharmacokinetics, polyuria in rats". British Journal of Pharmacology 56 (4): 399–402. April 1976. doi:10.1111/j.1476-5381.1976.tb07449.x. PMID 1260219.
- ↑ "Rat brain and serum lithium concentrations after acute injections of lithium carbonate and orotate". The Journal of Pharmacy and Pharmacology 30 (6): 368–370. June 1978. doi:10.1111/j.2042-7158.1978.tb13258.x. PMID 26768.
- ↑ "Kidney function and lithium concentrations of rats given an injection of lithium orotate or lithium carbonate". The Journal of Pharmacy and Pharmacology 31 (3): 161–163. March 1979. doi:10.1111/j.2042-7158.1979.tb13461.x. PMID 34690.
- ↑ 8.0 8.1 8.2 "Lithium orotate: A superior option for lithium therapy?". Brain and Behavior 11 (8): e2262. August 2021. doi:10.1002/brb3.2262. PMID 34196467.
- ↑ Pacholko AG, Bekar LK (2022-05-01). "Lithium orotate is more potent, effective, and less toxic than lithium carbonate in a mouse model of mania". bioRxiv 10.1101/2022.05.01.490227.
- ↑ "Low dosage lithium augmentation in venlafaxine resistant depression: an open-label study". Psychiatrike = Psychiatriki 23 (2): 143–148. 2012. PMID 22796912.
- ↑ "Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer's disease". Current Alzheimer Research 10 (1): 104–107. January 2013. doi:10.2174/156720513804871354. PMID 22746245.
- ↑ "Neuroprotective effects of low-dose lithium in individuals at ultra-high risk for psychosis. A longitudinal MRI/MRS study". Current Pharmaceutical Design 18 (4): 570–575. 2012. doi:10.2174/138161212799316163. PMID 22239590.
- ↑ "Possible dangers of a "nutritional supplement" lithium orotate". Annals of Clinical Psychiatry 25 (1): 71. February 2013. PMID 23376874.
- ↑ 14.0 14.1 "Lithium toxicity from an Internet dietary supplement". Journal of Medical Toxicology 3 (2): 61–62. June 2007. doi:10.1007/bf03160910. PMID 18072162.
- ↑ "Adverse Consequences of Internet Purchase of Pharmacologic Agents or Dietary Supplements". Journal of Pharmacy Technology 25 (6): 355–360. 1 November 2009. doi:10.1177/875512250902500602.
- ↑ "Lithium Toxicity". StatPearls. Treasure Island (FL): StatPearls Publishing. 2023. http://www.ncbi.nlm.nih.gov/books/NBK499992/. Retrieved 2023-04-13.
- ↑ "Vitamin B13 cannot be proven safe, says EFSA" (in en-GB). nutraingredients.com. https://www.nutraingredients.com/Article/2009/07/30/Vitamin-B13-cannot-be-proven-safe-says-EFSA#.
- ↑ "Material Safety Data Sheet - Orotic Acid, anhydrous MSDS". 2013-05-21. http://www.sciencelab.com/msds.php?msdsId=9926339.
- ↑ "A toxicological evaluation of lithium orotate". Regulatory Toxicology and Pharmacology 124: 104973. August 2021. doi:10.1016/j.yrtph.2021.104973. PMID 34146638.
External links
- "Ask the Mental Health Expert". Ron Pies, M.D., clinical professor of psychiatry at Tufts University. December 2002. http://www.healthieryou.com/mhexpert/exp1121602b.html.
 |

