Biology:FAN1
 Generic protein structure example |
FANCD2/FANCI-associated nuclease 1 (KIAA1018) is an enzyme that in humans is encoded by the FAN1 gene. It is a structure dependent endonuclease. It is thought to play an important role in the Fanconi Anemia (FA) pathway.[1]
Structure
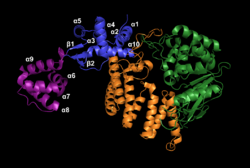
FAN1 is a protein of 1017 amino acids.[3] Several crystal structures of the residues 373-1017 have been characterized. This portion of FAN1 contains three domains: an SAP domain (primary-DNA binding domain), a TPR domain (mediating interdomain interaction and dimerization interface) and the virus-type replication-repair nuclease module (VRR_NUC, catalytic site) (Figure 1).[4] DNA binding promotes dimerization of FAN1 in a "head to tail" fashion.[2]
The SAP region contains three major components: α9, α5β1, and α7. The core helix α9 stabilizes the protein as it moves through dimer configurations and mediates the interactions between α5β1 and α7 as they adjust their positions. These three configurations are the substrate scanning, substrate latching and substrate unwinding forms (figure 2).[2]
In the FAN1 dimer, the SAP regions of both FAN1 enzymes make contact with the DNA duplex (dsDNA). This double contact facilitates DNA induced dimerization, as well as guiding the single stranded (ssDNA) into the SAP domain of the downstream enzyme (PSAP). The SAP domain of the upstream FAN1 component enzyme (ASAP) aids in guiding the DNA to PSAP.[2]
The SAP surface facing the catalytic site is the most conserved region between FAN1 homologs. It is positively charged for favorable hydrogen bonding and electrostatic interactions with DNA. In particular, residues Y374 and Y436 form hydrogen bonds with the phosphate backbone. FAN1 can bind DNA in either direction. However, when the 5' flab is facing away from the VRR_NUC site, substrate latching and unwinding cannot occur.[2] The unresolved portion of FAN1 contains a Zinc finger at the N terminus called a UBZ region. This is present in proteins that bind to ubiquitinated proteins, and is highly conserved across eukaryotes. This Zinc finger is crucial for recruitment to the ubiquitinated FANCD2/FANCI complex, and is found in other nucleases.[3] The VRR_Nuc catalytic domain is located at the C terminus and contains the endonuclease functionality.[3] FAN1 is the first known instance of a virus type replication-repair nuclease module in eukaryotes. It is normally found as a standalone domain in bacterial and viral Holliday Junction Resolvases (HJR). FAN1 does not exhibit any activity on Holliday Junction (HJ) substrates.[4] A subdomain of SAP consisting of six α helices connected to the VRR_Nuc region is thought to inhibit HJR activity.[5]
Function

Interstrand DNA crosslinks (ICLs) effectively block the progression of transcription and replication machineries. Release of this block, referred to as unhooking, is thought to require incision of one strand of the duplex on either side of the ICL.
Repair of interstrand DNA crosslinks is triggered when the DNA replication fork is unable to continue. The FA proteins play an elaborate role with FAN1 to remove these ICLs. The pathway consists of 15 known proteins. Three of them form the FA AP24-MHF1/2 complex which recognizes the ICL (from stalled replication forks). This recruits the FA core complex, which consists of 8 proteins. This complex monoubiquitinates FANCD2 and FANCI, which allows it to form a heterodimer. It is this complex that recruits FAN1 as well as other nucleases such as SLX4.[5] Ubiquinated FANCD2 interacts with the FAN1 nuclease. Upon its recruitment by FANCD2, FAN1 acts to restrain DNA replication fork progression and to prevent chromosome abnormalities from occurring when DNA replication forks stall.[7] FAN1 is typically localized in the nucleus, but forms very distinct loci at damaged regions when ICLs are present.[8]
The FAN1 protein possesses endonuclease and exonuclease functions to remove ICLs. At a replication fork arrested at an ICL, FAN1 nuclease action can catalyze incisions in the double-stranded region.[9] It is thought that this process consists of unhooking the crosslink and separating the DNA strands through two incision events, yielding one strand with a crosslinked nucleotide and another strand with a gap.[10][11] FAN1 preferentially acts as a 5’ flap endonuclease. This is illustrated in Figure 2, which shows the sequence of substrate scanning, latching, and unwinding. It usually cleaves about 5 nucleotides from a junction. FAN1 will also incise at splayed arms, three way junctions, and 3’ flaps (in order of decreasing preference). In high concentrations FAN1 has been shown to exhibit 3’ 5’ exonuclease activity. In blunt end substrates, FAN1 has also 5’ recessed ends. However, FAN1 does not appear to bind to single stranded DNA.[3][12]
The presence of the FANCD2/FANCI complex is unaffected by knockdown of FAN1. This is because FAN1 acts downstream to the recruitment of FANCD2/FANCI.[2][3][13] FAN1 has also been shown to increase the frequency of homologous recombination.[3] This suggests that the gapped intermediate that forms following ICL unhooking may be repaired through HR when homologous chromosomes are present.[12] FAN1 does not appear to be involved in other types of DNA repair, as it does not localize to DNA upon irradiation.[8]
Clinical significance
Mutations affecting the function of the 15 known FA genes are associated with Fanconi anemia, a recessive autosomal disorder.[13] It is characterized by congenital abnormalities as well as anemia, bone marrow failure, and cancer predisposition in childhood.[5] However, some patients have “unassigned” Fanconi Anemia where no mutations in the known FA genes can be found. Mutations in FAN1 can result in chronic kidney diseases and neurological conditions such as schizophrenia.[2][14] However, recent research has called into question the categorization of FAN1 as an FA gene. In 2015 researchers studied four individuals with chromosomal microdeletion of 15q13.3. Analysis of blood samples revealed only mild ICL agent sensitivity and chromosomal fragility consistent with Fanconi Anemia.[15]
A deficiency of FAN1 increases in vitro sensitivity to cisplatin and mitomycin C, two crosslinking agents[2][3] FAN1 is also able to repair mitomycin C induced double strand breaks.[3]
Germline mutations in the FAN1 gene can cause hereditary colorectal cancer due to defective DNA repair.[16]
References
- ↑ "Entrez Gene: FANCD2/FANCI-associated nuclease 1". https://www.ncbi.nlm.nih.gov/gene/22909.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Structural insights into 5' flap DNA unwinding and incision by the human FAN1 dimer". Nature Communications 5 (11): 5726. December 2014. doi:10.1038/ncomms6726. PMID 25500724. Bibcode: 2014NatCo...5.5726Z.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Identification of KIAA1018/FAN1, a DNA repair nuclease recruited to DNA damage by monoubiquitinated FANCD2". Cell 142 (1): 65–76. July 2010. doi:10.1016/j.cell.2010.06.021. PMID 20603015.
- ↑ 4.0 4.1 "FAN1 activity on asymmetric repair intermediates is mediated by an atypical monomeric virus-type replication-repair nuclease domain". Cell Reports 8 (1): 84–93. July 2014. doi:10.1016/j.celrep.2014.06.001. PMID 24981866.
- ↑ 5.0 5.1 5.2 "Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway". Genes & Development 26 (13): 1393–408. July 2012. doi:10.1101/gad.195248.112. PMID 22751496.
- ↑ "DNA repair. Mechanism of DNA interstrand cross-link processing by repair nuclease FAN1". Science 346 (6213): 1127–30. November 2014. doi:10.1126/science.1258973. PMID 25430771.
- ↑ "Ubiquitinated Fancd2 recruits Fan1 to stalled replication forks to prevent genome instability". Science 351 (6275): 846–9. February 2016. doi:10.1126/science.aad5634. PMID 26797144. Bibcode: 2016Sci...351..846L.
- ↑ 8.0 8.1 "Human KIAA1018/FAN1 localizes to stalled replication forks via its ubiquitin-binding domain". Cell Cycle 9 (19): 3977–83. October 2010. doi:10.4161/cc.9.19.13207. PMID 20935496.
- ↑ "FANCD2-associated nuclease 1, but not exonuclease 1 or flap endonuclease 1, is able to unhook DNA interstrand cross-links in vitro". The Journal of Biological Chemistry 290 (37): 22602–11. September 2015. doi:10.1074/jbc.M115.663666. PMID 26221031.
- ↑ "A genetic screen identifies FAN1, a Fanconi anemia-associated nuclease necessary for DNA interstrand crosslink repair". Molecular Cell 39 (1): 36–47. July 2010. doi:10.1016/j.molcel.2010.06.023. PMID 20603073.
- ↑ "Expanded roles of the Fanconi anemia pathway in preserving genomic stability". Genes & Development 24 (16): 1680–94. August 2010. doi:10.1101/gad.1955310. PMID 20713514.
- ↑ 12.0 12.1 "Orchestrating the nucleases involved in DNA interstrand cross-link (ICL) repair". Cell Cycle 10 (23): 3999–4008. December 2011. doi:10.4161/cc.10.23.18385. PMID 22101340.
- ↑ 13.0 13.1 "FAN1 acts with FANCI-FANCD2 to promote DNA interstrand cross-link repair". Science 329 (5992): 693–6. August 2010. doi:10.1126/science.1192656. PMID 20671156. Bibcode: 2010Sci...329..693L.
- ↑ "FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair". Nature Genetics 44 (8): 910–5. July 2012. doi:10.1038/ng.2347. PMID 22772369.
- ↑ "On the role of FAN1 in Fanconi anemia". Blood 120 (1): 86–9. July 2012. doi:10.1182/blood-2012-04-420604. PMID 22611161.
- ↑ "Germline Mutations in FAN1 Cause Hereditary Colorectal Cancer by Impairing DNA Repair". Gastroenterology 149 (3): 563–6. September 2015. doi:10.1053/j.gastro.2015.05.056. PMID 26052075.
Further reading
- "KIAA1018/FAN1 nuclease protects cells against genomic instability induced by interstrand cross-linking agents". Proceedings of the National Academy of Sciences of the United States of America 107 (50): 21553–7. December 2010. doi:10.1073/pnas.1011081107. PMID 21115814. Bibcode: 2010PNAS..10721553Y.
- "FAN1 variants identified in multiple-case early-onset breast cancer families via exome sequencing: no evidence for association with risk for breast cancer". Breast Cancer Research and Treatment 130 (3): 1043–9. December 2011. doi:10.1007/s10549-011-1704-y. PMID 21858661.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |

