Biology:Opsin


Animal opsins are G-protein-coupled receptors and a group of proteins made light-sensitive via a chromophore, typically retinal. When bound to retinal, opsins become retinylidene proteins, but are usually still called opsins regardless. Most prominently, they are found in photoreceptor cells of the retina. Five classical groups of opsins are involved in vision, mediating the conversion of a photon of light into an electrochemical signal, the first step in the visual transduction cascade. Another opsin found in the mammalian retina, melanopsin, is involved in circadian rhythms and pupillary reflex but not in vision. Humans have in total nine opsins. Beside vision and light perception, opsins may also sense temperature, sound, or chemicals.
Structure and function
Animal opsins detect light and are the molecules that allow us to see. Opsins are G-protein-coupled receptors (GPCRs),[1][2] which are chemoreceptors and have seven transmembrane domains forming a binding pocket for a ligand.[3][4] The ligand for opsins is the vitamin A-based chromophore 11-cis-retinal,[5][6][7][8][9] which is covalently bound to a lysine residue[10] in the seventh transmembrane domain[11][12][13] through a Schiff-base.[14][15] However, 11-cis-retinal only blocks the binding pocket and does not activate the opsin. The opsin is only activated when 11-cis-retinal absorbs a photon of light and isomerizes to all-trans-retinal,[16][17] the receptor activating form,[18][19] causing conformal changes in the opsin,[18] which activate a phototransduction cascade.[20] Thus, a chemoreceptor is converted to a light or photo(n)receptor.[21]
In the vertebrate photoreceptor cells, all-trans-retinal is released and replaced by a newly synthesized 11-cis-retinal provided from the retinal epithelial cells. Beside 11-cis-retinal (A1), 11-cis-3,4-didehydroretinal (A2) is also found in vertebrates as ligand such as in freshwater fishes.[19] A2-bound opsins have a shifted λmax and absorption spectrum compared to A1-bound opsins.[22]
Functionally conserved residues and motifs
The seven transmembrane α-helical domains in opsins are connected by three extra-cellular and three cytoplasmic loops. Along the α-helices and the loops, many amino acid residues are highly conserved between all opsin groups, indicating that they serve important functions and thus are called functionally conserved residues. Actually, insertions and deletions in the α-helices are very rare and should preferentially occur in the loops. Therefore, different G-protein-coupled receptors have different length and homologous residues may be in different positions. To make such positions comparable between different receptors, Ballesteros and Weinstein introduced a common numbering scheme for G-protein-coupled receptors.[23] The number before the period is the number of the transmembrane domain. The number after the period is set arbitrarily to 50 for the most conserved residue in that transmembrane domain among GPCRs known in 1995. For instance in the seventh transmembrane domain, the proline in the highly conserved NPxxY7.53 motif is Pro7.50, the asparagine before is then Asp7.49, and the tyrosine three residues after is then Tyr7.53.[21] Another numbering scheme is based on cattle rhodopsin. Cattle rhodopsin has 348 amino acids and is the first opsin whose amino acid sequence[24] and whose 3D-structure were determined.[12] The cattle rhodopsin numbering scheme is widespread in the opsin literature.[21] Therefore, it is useful to use both schemes.
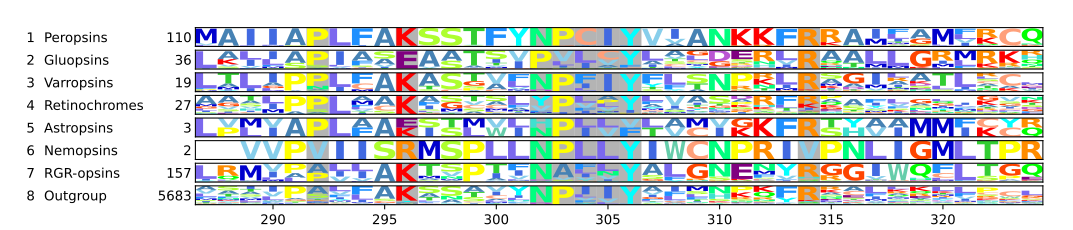
The retinal binding lysine
Opsins without the retinal binding lysine are not light sensitive.[25][26] In cattle rhodopsin, this lysine is the 296th amino acid[12][24] and thus according to both numbering schemes Lys2967.43. It is well conserved among opsins, so well conserved that sequences without it were not even considered opsins and thus excluded from large scale phylogenetic reconstructions.[27][28] Even so most opsins have Lys2967.43, some have lost it during evolution: In the nemopsins from nematodes, Lys2967.43 is replaced by Arginine.[29][21] In the astropsins from sea urchins[30][21] and in the gluopsins, Lys2967.43 is replaced by glutamic acid.[21] A nemopsin is expressed in chemosensory cells in Caenorhabditis elegans. Therefore, the nemopsins are thought to be chemoreceptors.[29] The gluopsins are found in insects such as beetles, scorpionflies, dragonflies, and butterflies and moths including model organisms such as the silk moth and the tobacco hawk moth. However, the gluopsins have no known function.[21]
Such function does not need to be light detection, as some opsins are also involved in thermosensation,[31] mechanoreception such as hearing[32] detecting phospholipids, chemosensation, and other functions.[33][34] In particular, the Drosophila rhabdomeric opsins (rhabopsins, r-opsins) Rh1, Rh4, and Rh7 function not only as photoreceptors, but also as chemoreceptors for aristolochic acid. These opsins still have Lys2967.43 like other opsins. However, if this lysine is replaced by an arginine in Rh1, then Rh1 loses light sensitivity but still responds to aristolochic acid. Thus, Lys2967.43 is not needed for Rh1 to function as chemoreceptor.[26] Also the Drosophila rhabopsins Rh1 and Rh6 are involved in mechanoreception, again for mechanoreception Lys2967.43 is not needed, but needed for proper function in the photoreceptor cells.[25]
Beside these functions, an opsin without Lys2967.43, such as a gluopsin, could still be light sensitive, since in cattle rhodopsin, the retinal binding lysine can be shifted from position 296 to other positions, even into other transmembrane domains, without changing light sensitivity.[35]
-
Most known opsins have the retinal binding lysine except some among the tetraopins, The outgroup contains other G protein-coupled receptors.
-
Most tetraopsins have also the retinal binding lysine except some of the chromopsins, which are highlighted by the frame and expanded in the next image. The outgroup contains other G protein-coupled receptors including the other opsins.
-
Most chromopsins have also the retinal binding lysine except the nemopsins, where it is replaced by argenine (R), and the gluopsins, where it is replaced by glutamic acid (E). The astropsins, the nemopsins and the gluopsins are highlighted by the frames. The outgroup contains other G protein-coupled receptors including the other opsins.
In the phylogeny above, Each clade contains sequences from opsins and other G protein-coupled receptors. The number of sequences and two pie charts are shown next to the clade. The first pie chart shows the percentage of a certain amino acid at the position in the sequences corresponding Lys2967.43 in cattle rhodopsin. The amino acids are color-coded. The colors are red for lysine (K), purple for glutamic acid (E), orange for argenine (R), dark and mid-gray for other amino acids, and light gray for sequences that have no data at that position. The second pie chart gives the taxon composition for each clade, green stands for craniates, dark green for cephalochordates, mid green for echinoderms, brown for nematodes, pale pink for annelids, dark blue for arthropods, light blue for mollusks, and purple for cnidarians. The branches to the clades have pie charts, which give support values for the branches. The values are from right to left SH-aLRT/aBayes/UFBoot. The branches are considered supported when SH-aLRT ≥ 80%, aBayes ≥ 0.95, and UFBoot ≥ 95%. If a support value is above its threshold the pie chart is black otherwise gray.[21]
The NPxxY motif
The NPxxY7.53 motif is well-conserved among opsins and G-protein-coupled receptors. This motif is important for G-protein binding and receptor activation.[21] For instance, if it is mutated to DPxxY7.53 (Asn7.49 → Asp7.49) in the human m3 muscarinic receptor, activation is not affected, but it is abolished if it is mutated to APxxY7.53 (Asn7.49 → Ala7.49).[36] Such a mutation to APxxY7.53 (Asn7.49 → Ala7.49) reduces the G-protein activation of cattle rhodopsin to 45% compared to wild type. Also in cattle rhodopsin, if the motif is mutated to NPxxA7.53 (Tyr7.53 → Ala7.53), cattle rhodopsin does not activate the G-protein.[37] Such a mutation also reduces the activation of the vasopressin V2 receptor. In fact in G-protein-coupled receptors, only loss of function disease mutations are known for Tyr7.53.[38]
Also mutations of Pro7.50 influence G-protein activation, if the motif is mutated to NAxxY7.53 (Pro7.50 → Ala7.50) in the rat m3 muscarinic receptor, the receptor can still be activated but less efficiently,[39] this mutation even abolishes activation in the cholecystokinin B receptor completely.[40] In fact, the RGR-opsins have NAxxY7.53 and retinochromes have VPxxY7.53 for annelids or YPxxY7.53 for mollusks, natively. Both RGR-opsins and retinochromes, belong to the chromopsins.[21] RGR-opsins[41] and retinochromes[42] also bind unlike most opsins all-trans-retinal in the dark and convert it to 11-cis-retinal when illuminated. Therefore, RGR-opsins and retinochromes are thought to neither signal nor activate a phototransduction cascade but to work as photoisomerases to produce 11-cis-retinal for other opsins.[43][44] This view is considered established in the opsin literature,[34][45][43][46][47] even so it has not been shown, conclusively.[21] In fact, the human MT2 melatonin receptor signals via a G-protein and has an NAxxY7.53 motif natively. If this motif is mutated to NPxxY7.53 (Ala7.50 → Pro7.50), the receptor cannot be activated, but can be rescued partially if the motif is mutated to NVxxY7.53 (Ala7.50 → Val7.50).[48] Furthermore, when the motif is mutated to NAxxY7.53 (Pro7.50 → Ala7.50) in cattle rhodopsin, the mutant has 141% of wild type activity.[37] This evidence shows that a GPCR does not need a standard NPxxY7.53 motif for signaling.[21]
Other residues and motifs
Cys138 and Cys110 form a highly conserved disulfide bridge. Glu113 serves as the counterion, stabilizing the protonation of the Schiff linkage between Lys296 and the ligand retinal. The Glu134-Arg135-Tyr136 is another highly conserved motif, involved in the propagation of the transduction signal once a photon has been absorbed.
Spectral tuning sites
Certain amino acid residues, termed spectral tuning sites, have a strong effect on λmax values. Using site-directed mutagenesis, it is possible to selectively mutate these residues and investigate the resulting changes in light absorption properties of the opsin. It is important to differentiate spectral tuning sites, residues that affect the wavelength at which the opsin absorbs light, from functionally conserved sites, residues important for the proper functioning of the opsin. They are not mutually exclusive, but, for practical reasons, it is easier to investigate spectral tuning sites that do not affect opsin functionality. For a comprehensive review of spectral tuning sites see Yokoyama[49] and Deeb.[50] The impact of spectral tuning sites on λmax differs between different opsin groups and between opsin groups of different species.
Opsins in the human eye, brain, and skin
| Abbr. | Name | λmax | Color | Eye | Brain | Skin | Chromosomal location[44] |
|---|---|---|---|---|---|---|---|
| OPN1LW | L-cone (red-cone) opsin | 557 nm | Yellow | Cone | N/A | N/A | Xq28[44] |
| OPN1MW | M-cone (green-cone) opsin | 527 nm | Green | Cone | N/A | N/A | Xq28[44] |
| OPN1SW | S-cone (blue-cone) opsin | 420 nm | Violet | Cone | N/A | Melanocytes, keratinocytes[51] | 7q32.1[44] |
| OPN2 (RHO) | Rhodopsin | 505 nm | Blue–green | Rod | N/A | Melanocytes, keratinocytes[51] | 3q22.1[44] |
| OPN3 | Encephalopsin, panopsin | S-M | Blue–green | Rod, cone, OPL, IPL, GCL[52] | Cerebral cortex, cerebellum, striatum, thalamus, hypothalamus[53][54] | Melanocytes, keratinocytes[51] | 1q43[44] |
| OPN4 | Melanopsin | 480 nm[55] | Sky blue | ipRGC[55] | N/A | N/A | 10q23.2[44] |
| OPN5 | Neuropsin | 380 nm[56] | Ultraviolet[56] | Neural retina, RPE[57] | Anterior hypothalamus[58] | Melanocytes, keratinocytes[51] | 6p12.3[44] |
| RRH | Peropsin | RPE cells - microvilli | N/A | N/A | 4q25[44] | ||
| RGR | Retinal G protein coupled receptor | RPE cells | N/A | N/A | 10q23.1[44] |
RPE, retinal pigment epithelium; ipRGC, intrinsically photosensitive retinal ganglion cells; OPL, outer plexiform layer; IPL, inner plexiform layer; GCL, ganglion cell layer
Cuttlefish
Cuttlefish and octopuses contain opsin in their skin as part of the chromophores. The opsin is part of the sensing network detecting the colour and shape of the cuttlefish's surroundings.[59][60][61]
Phylogeny
Animal opsins (also known as type 2 opsins) are members of the seven-transmembrane-domain proteins of the G protein-coupled receptor (GPCR) superfamily.[1][2]
Animal opsins fall phylogenetically into five groups: The ciliary opsins (cilopsins, c-opsins), the rhabdomeric opsins (r-opsins, rhabopsins), the xenopsins, the nessopsins, and the tetraopsins. Four of these subclades occur in Bilateria (all but the nessopsins).[21][28] However, the bilaterian clades constitute a paraphyletic taxon without the opsins from the cnidarians.[21][28][27][62] The nessopsins are also known as anthozoan opsins II[63] or simply as the cnidarian opsins.[64] The tetraopsins are also known as RGR/Go[65] or Group 4 opsins[27] and contain three subgroups: the neuropsins, the Go-opsins, and the chromopsins.[21][28][64] The chromopsins have seven subgroups: the RGR-opsins, the retinochromes, the peropsins, the varropsins, the astropsins, the nemopsins, and the gluopsins.[21]
Animal visual opsins are traditionally classified as either ciliary or rhabdomeric. Ciliary opsins, found in vertebrates and cnidarians, attach to ciliary structures such as rods and cones. Rhabdomeric opsins are attached to light-gathering organelles called rhabdomeres. This classification cuts across phylogenetic categories (clades) so that both the terms "ciliary" and "rhabdomeric" can be ambiguous. Here, "C-opsins (ciliary)" refers to a clade found exclusively in Bilateria and excludes cnidarian ciliary opsins such as those found in the box jellyfish. Similarly, "R-opsin (rhabdomeric)" includes melanopsin even though it does not occur on rhabdomeres in vertebrates.[27]
Ciliary opsins
Ciliary opsins (cilopsins, c-opsins) are expressed in ciliary photoreceptor cells, and include the vertebrate visual opsins and encephalopsins.[66] They convert light signals to nerve impulses via cyclic nucleotide gated ion channels, which work by increasing the charge differential across the cell membrane (i.e. hyperpolarization.[67])
Vertebrate visual opsins
Vertebrate visual opsins are a subclass of ciliary opsins that express in the vertebrate retina and mediate vision. They are further subdivided into:
- Photopsins - those responsible for photopic vision (daylight), which are expressed in cone cells; hence also cone opsins. Photopsins are further subdivided according to their spectral sensitivity, namely the wavelength at which the highest light absorption is observed (λmax). Vertebrates generally have four (SWS1, SWS2, RH2, LWS) classes of photopsins.[68][69] Mammals lost Rh2 and SWS2 classes during the nocturnal bottleneck, so are generally dichromatic. Primate ancestors later developed two distinct LWS opsins (LWS and MWS), leaving humans with 3 photopsins in 2 classes: SWS1 (OPN1SW) and two forms of LWS (OPN1LW, OPN1MW).
- Scotopsins - those responsible for scotopic vision (dim light), which are expressed in rod cells; hence also rod opsins.[66] The most common form of scotopsin is rhodopsin, thus usually denoted Rh1.[70]
Extraretinal (or extra-ocular) Rhodopsin-Like Opsins (Exo-Rh)
These pineal opsins, found in the Actinopterygii (ray-finned fish) apparently arose as a result of gene duplication from Rh1 (rhodopsin). These opsins appear to serve functions similar to those of pinopsin found in birds and reptiles.[71] [72]
Pinopsins
The first Pineal Opsin (Pinopsin) was found in the chicken pineal gland. It is a blue sensitive opsin (λmax = 470 nm).[73][74]
Pineal opsins have a wide range of expression in the brain, most notably in the pineal region.
Vertebrate Ancient (VA) opsin
Vertebrate Ancient (VA) opsin has three isoforms VA short (VAS), VA medium (VAM), and VA long (VAL). It is expressed in the inner retina, within the horizontal and amacrine cells, as well as the pineal organ and habenular region of the brain.[75] It is sensitive to approximately 500 nm [14], found in most vertebrate classes, but not in mammals.[76]
Parapinopsins
The first parapinopsin (PP) was found in the parapineal organ of the catfish.[77] The parapinopsin of lamprey is a UV-sensitive opsin (λmax = 370 nm).[78] The teleosts have two groups of parapinopsins, one is sensitive to UV (λmax = 360-370 nm), the other is sensitive to blue (λmax = 460-480 nm) light.[79]
Parietopsins
The first parietopsin was found in the photoreceptor cells of the lizard parietal eye. The lizard parietopsin is green-sensitive (λmax = 522 nm), and despite it is a c-opsin, like the vertebrate visual opsins, it does not induce hyperpolarization via a Gt-protein, but induces depolarization via a Go-protein.[80][81]
Encephalopsin or Panopsin
The panopsins are found in many tissues (skin,[51] brain,[53][82] testes,[53] heart, liver,[82] kidney, skeletal muscle, lung, pancreas and retina[82]). They were originally found in the human and mouse brain and thus called encephalopsin.[53]
The first invertebrate panopsin was found in the ciliary photoreceptor cells of the annelid Platynereis dumerilii and is called c(iliary)-opsin.[83] This c-opsin is UV-sensitive (λmax = 383 nm) and can be tuned by 125 nm at a single amino-acid (range λmax = 377 - 502 nm).[84] Thus, not unsurprisingly, a second but cyan sensitive c-opsin (λmax = 490 nm) exists in Platynereis dumerilii.[85] The first c-opsin mediates in the larva UV induced gravitaxis. The gravitaxis forms with phototaxis a ratio-chromatic depth-gauge.[86] In different depths, the light in water is composed of different wavelengths: First the red (> 600 nm) and the UV and violet (< 420 nm) wavelengths disappear. The higher the depth the narrower the spectrum so that only cyan light (480 nm) is left.[87] Thus, the larvae can determine their depth by color. The color unlike brightness stays almost constant independent of time of day or the weather, for instance if it is cloudy.[88][89]
Panopsins are also expressed in the brains of some insects.[66] The panopsins of mosquito and pufferfish absorb maximally at 500 nm and 460 nm, respectively. Both activate in vitro Gi and Go proteins.[90]
The panopsins are sister to the TMT-opsins.[28][91][47][92]
Teleost Multiple Tissue (TMT) Opsin
The first TMT-opsin was found in many tissues in Teleost fish and therefore they are called Teleost Multiple Tissue (TMT) opsins.[93] TMT-opsins form three groups which are most closely related to a fourth group the panopsins, which thus are paralogous to the TMT-opsins.[28][47][91][92] TMT-opsins and panopsins also share the same introns, which confirms that they belong together.[93]
Opsins in cnidarians
Cnidaria, which include jellyfish, corals, and sea anemones, are the most basal animals to possess complex eyes. Jellyfish opsins in the rhopalia couple to Gs-proteins raising the intracellular cAMP level.[94][62] Coral opsins can couple to Gq-proteins and Gc-proteins. Gc-proteins are a subtype of G-proteins specific to cnidarians.[95] The cnidarian opsins belong to two groups the xenopsins and the nessopsins. The xenopsins contain also bilaterian opsins, while the nessopsins are restricted to the cnidarians.[21][28] However, earlier studies have found that some cnidarian opsins belong to the cilopsins, rhabopsins, and the tetraopsins of the bilaterians.[65][96][97]
Rhabdomeric opsins
Rhabdomeric opsins (rhabopsins, r-opsins) are also known as Gq-opsins, because they couple to a Gq-protein. Rhabopsins are used by molluscs and arthropods. Arthropods appear to attain colour vision in a similar fashion to the vertebrates, by using three (or more) distinct groups of opsins, distinct both in terms of phylogeny and spectral sensitivity.[66] The rhabopsin melanopsin is also expressed in vertebrates, where it regulates circadian rhythms and mediates the pupillary reflex.[66]
Unlike cilopsins, rhabopsins are associated with canonical transient receptor potential ion channels; these lead to the electric potential difference across a cell membrane being eradicated (i.e. depolarization).[67]
The identification of the crystal structure of squid rhodopsin[13] is likely to further our understanding of its function in this group.
Arthropods use different opsins in their different eye types, but at least in Limulus the opsins expressed in the lateral and the compound eyes are 99% identical and presumably diverged recently.[98]
Melanopsin
Melanopsin (OPN4) is involved in circadian rhythms, the pupillary reflex, and color correction in high-brightness situations. Phylogenetically, it is a member of the rhabdomeric opsins (rhabopsins, r-opsins) and functionally and structurally a rhabopsin, but does not occur in rhabdomeres.
Tetraopsins
The tetraopsins include the neuropsins, the Go-opsins, and the chromopsins.[21][28][64] The chromopsins consist of seven subgroups: the RGR-opsins, the retinochromes, the peropsins, the varropsins, the astropsins, the nemopsins, and the gluopsins.[21]
Neuropsins
Neuropsins are sensitive to UVA, typically at 380 nm. They are found in the brain, testes, skin, and retina of humans and rodents, as well as in the brain and retina of birds. In birds and rodents they mediate ultraviolet vision.[51][56][99] They couple to Gi-proteins.[56][99] In humans, Neuropsin is encoded by the OPN5 gene. In the human retina, its function is unknown. In the mouse, it photo-entrains the retina and cornea at least ex vivo.[100]
Go-opsins
Go-opsins are absent from higher vertebrates[27] and ecdysozoans.[101] They are found in the ciliary photoreceptor cells of the scallop eye[102] and the basal chordate amphioxus.[103] In Platynereis dumerilii however, a Go-opsin is expressed in the rhabdomeric photoreceptor cells of the eyes.[87]
RGR-opsins
RGR-opsins, also known as Retinal G protein coupled receptors are expressed in the retinal pigment epithelium (RPE) and Müller cells.[104] They preferentially bind all-trans-retinal in the dark instead of 11-cis-retinal.[41] RGR-opsins were thought to be photoisomerases[44] but instead, they regulate retinoid traffic and production.[66][105] In particular, they speed up light-independently the production of 11-cis-retinol (a precursor of 11-cis-retinal) from all-trans-retinyl-esters.[106] However, the all-trans-retinyl-esters are made available light-dependently by RGR-opsins. Whether RGR-opsins regulate this via a G-protein or another signaling mechanism is unknown.[107] The cattle RGR-opsin absorbs maximally at different wavelengths depending on the pH-value. At high pH it absorbs maximally blue (469 nm) light and at low pH it absorbs maximally UV (370 nm) light.[108]
Peropsin
Peropsin, a visual pigment-like receptor, is a protein that in humans is encoded by the RRH gene.[109]
Other proteins called opsins
Photoreceptors can be classified several ways, including function (vision, phototaxis, photoperiodism, etc.), type of chromophore (retinal, flavine, bilin), molecular structure (tertiary, quaternary), signal output (phosphorylation, reduction, oxidation), etc.[110]
Beside animal opsins, which are G protein-coupled receptors, there is another group of photoreceptor proteins called opsins.[67][111] These are the microbial opsin, they are used by prokaryotes and by some algae (as a component of channelrhodopsins) and fungi,[112] whereas animals use animal opsins, exclusively. No opsins have been found outside these groups (for instance in plants, or placozoans).[67]
Microbial and animal opsins are also called type 1 and type 2 opsins respectively. Both types are called opsins, because at one time it was thought that they were related: Both are seven-transmembrane receptors and bind covalently retinal as chromophore, which turns them into photoreceptors sensing light. However, both types are not related on the sequence level.[113]
In fact, the sequence identity between animal and mirobial opsins is no greater than could be accounted for by random chance. However, in recent years new methods have been developed specific to deep phylogeny. As a result, several studies have found evidence of a possible phylogenetic relationship between the two.[114][35][115] However, this does not necessarily mean that the last common ancestor of microbial and animal opsins was itself light sensitive: All animal opsins arose (by gene duplication and divergence) late in the history of the large G-protein coupled receptor (GPCR) gene family, which itself arose after the divergence of plants, fungi, choanflagellates and sponges from the earliest animals. The retinal chromophore is found solely in the opsin branch of this large gene family, meaning its occurrence elsewhere represents convergent evolution, not homology. Microbial rhodopsins are, by sequence, very different from any of the GPCR families.[116] According to one hypothesis, both microbial and animal opsins belong to the transporter-opsin-G protein-coupled receptor (TOG) superfamily, a proposed clade that includes G protein-coupled receptor (GPCR), Ion-translocating microbial rhodopsin (MR), and seven others.[117]
Most microbial opsins are ion channels or pumps instead of proper receptors and do not bind to a G protein. Microbal opsins are found in all three domains of life: Archaea, Bacteria, and Eukaryota. In Eukaryota, microbial opsins are found mainly in unicellular organisms such as green algae, and in fungi. In most complex multicellular eukaryotes, microbial opsins have been replaced with other light-sensitive molecules such as cryptochrome and phytochrome in plants, and animal opsins in animals.[118]
Microbial opsins are often known by the rhodopsin form of the molecule, i.e., rhodopsin (in the broad sense) = opsin + chromophore. Among the many kinds of microbial opsins are the proton pumps bacteriorhodopsin (BR) and xanthorhodopsin (xR), the chloride pump halorhodopsin (HR), the photosensors sensory rhodopsin I (SRI) and sensory rhodopsin II (SRII), as well as proteorhodopsin (PR), Neurospora opsin I (NOPI), Chlamydomonas sensory rhodopsins A (CSRA), Chlamydomonas sensory rhodopsins B (CSRB), channelrhodopsin (ChR), and archaerhodopsin (Arch).[119]
Several microbal opsins, such as proteo- and bacteriorhodopsin, are used by various bacterial groups to harvest energy from light to carry out metabolic processes using a non-chlorophyll-based pathway. Beside that, halorhodopsins of Halobacteria and channelrhodopsins of some algae, e.g. Volvox, serve them as light-gated ion channels, amongst others also for phototactic purposes. Sensory rhodopsins exist in Halobacteria that induce a phototactic response by interacting with transducer membrane-embedded proteins that have no relation to G proteins.[120]
Microbal opsins (like channelrhodopsin, halorhodopsin, and archaerhodopsin) are used in optogenetics to switch on or off neuronal activity. Microbal opsins are preferred if the neuronal activity should be modulated at higher frequency, because they respond faster than animal opsins. This is because microbal opsins are ion channels or proton/ion pumps and thus are activated by light directly, while animal opsins activate G-proteins, which then activate effector enzymes that produce metabolites to open ion channels.[121]
See also
- Retinylidene protein
- Visual cycle
- Visual phototransduction
- Microbial rhodopsin
- Channelrhodopsins
External links
- Illustration at Baldwin-Wallace College
- Opsin at the US National Library of Medicine Medical Subject Headings (MeSH)
References
- ↑ 1.0 1.1 "G protein involvement in receptor-effector coupling". The Journal of Biological Chemistry 263 (6): 2577–2580. February 1988. doi:10.1016/s0021-9258(18)69103-3. PMID 2830256.
- ↑ 2.0 2.1 "Fingerprinting G-protein-coupled receptors". Protein Engineering 7 (2): 195–203. February 1994. doi:10.1093/protein/7.2.195. PMID 8170923.
- ↑ "Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin". Nature 321 (6065): 75–79. May 1986. doi:10.1038/321075a0. PMID 3010132. Bibcode: 1986Natur.321...75D.
- ↑ "Ligand binding to the beta-adrenergic receptor involves its rhodopsin-like core". Nature 326 (6108): 73–77. March 1987. doi:10.1038/326073a0. PMID 2881211. Bibcode: 1987Natur.326...73D.
- ↑ "Carotenoids and the Vitamin A Cycle in Vision". Nature 134 (3376): 65. July 1934. doi:10.1038/134065a0. Bibcode: 1934Natur.134...65W.
- ↑ "Hindered Cis Isomers of Vitamin a and Retinene: The Structure of the Neo-B Isomer". Proceedings of the National Academy of Sciences of the United States of America 41 (7): 438–451. July 1955. doi:10.1073/pnas.41.7.438. PMID 16589696. Bibcode: 1955PNAS...41..438W.
- ↑ "The neo-b isomer of vitamin A and retinene". The Journal of Biological Chemistry 222 (2): 865–877. October 1956. doi:10.1016/S0021-9258(20)89944-X. PMID 13367054.
- ↑ "The Synthesis and Configuration of Neo-B Vitamin A and Neoretinine b". Journal of the American Chemical Society 78 (11): 2651–2652. June 1956. doi:10.1021/ja01592a095.
- ↑ "HINDERED CIS ISOMERS OF VITAMIN A AND RETINENE: THE STRUCTURE OF THE NEO-b ISOMER". Proceedings of the National Academy of Sciences of the United States of America 42 (9): 578–580. September 1956. doi:10.1073/pnas.42.9.578. PMID 16589909. Bibcode: 1956PNAS...42..578O.
- ↑ "Site of attachment of retinal in rhodopsin". Nature 216 (5121): 1178–1181. December 1967. doi:10.1038/2161178a0. PMID 4294735. Bibcode: 1967Natur.216.1178B.
- ↑ "The structure of bovine rhodopsin". Biophysics of Structure and Mechanism 9 (4): 235–244. 1983. doi:10.1007/BF00535659. PMID 6342691.
- ↑ 12.0 12.1 12.2 "Crystal structure of rhodopsin: A G protein-coupled receptor". Science 289 (5480): 739–745. August 2000. doi:10.1126/science.289.5480.739. PMID 10926528. Bibcode: 2000Sci...289..739P.
- ↑ 13.0 13.1 "Crystal structure of squid rhodopsin". Nature 453 (7193): 363–367. May 2008. doi:10.1038/nature06925. PMID 18480818. Bibcode: 2008Natur.453..363M.
- ↑ "Rhodopsin and indicator yellow". Nature 171 (4350): 469–471. March 1953. doi:10.1038/171469a0. PMID 13046517. Bibcode: 1953Natur.171..469C.
- ↑ "Studies on rhodopsin. VIII. Retinylidenemethylamine, an indicator yellow analogue". The Biochemical Journal 59 (1): 122–128. January 1955. doi:10.1042/bj0590122. PMID 14351151.
- ↑ "The Action of Light on Rhodopsin". Proceedings of the National Academy of Sciences of the United States of America 44 (2): 130–139. February 1958. doi:10.1073/pnas.44.2.130. PMID 16590155. Bibcode: 1958PNAS...44..130H.
- ↑ "The mechanism of bleaching rhodopsin". Annals of the New York Academy of Sciences 74 (2): 266–280. November 1959. doi:10.1111/j.1749-6632.1958.tb39550.x. PMID 13627857. Bibcode: 1959NYASA..74..266K.
- ↑ 18.0 18.1 "Crystal structure of metarhodopsin II". Nature 471 (7340): 651–655. March 2011. doi:10.1038/nature09789. PMID 21389988. Bibcode: 2011Natur.471..651C.
- ↑ 19.0 19.1 "Molecular basis of visual excitation". Science 162 (3850): 230–239. October 1968. doi:10.1126/science.162.3850.230. PMID 4877437. Bibcode: 1968Sci...162..230W.
- ↑ "Evolution and diversity of opsins". Wiley Interdisciplinary Reviews: Membrane Transport and Signaling 1 (1): 104–111. January 2012. doi:10.1002/wmts.6.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 21.10 21.11 21.12 21.13 21.14 21.15 21.16 21.17 21.18 21.19 21.20 "The Gluopsins: Opsins without the Retinal Binding Lysine". Cells 11 (15): 2441. August 2022. doi:10.3390/cells11152441. PMID 35954284.
 Material was copied and adapted from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied and adapted from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ "Spectral tuning of deep red cone pigments". Biochemistry 47 (16): 4614–4620. April 2008. doi:10.1021/bi702069d. PMID 18370404.
- ↑ "Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors". Methods in Neurosciences 25: 366–428. 1995. doi:10.1016/S1043-9471(05)80049-7.
- ↑ 24.0 24.1 Ovchinnikov, Yu.A. (November 1982). "Rhodopsin and bacteriorhodopsin: structure-function relationships". FEBS Letters 148 (2): 179–191. doi:10.1016/0014-5793(82)80805-3. PMID 6759163.
- ↑ 25.0 25.1 "Chromophore-Independent Roles of Opsin Apoproteins in Drosophila Mechanoreceptors". Current Biology 29 (17): 2961–2969.e4. September 2019. doi:10.1016/j.cub.2019.07.036. PMID 31447373.
- ↑ 26.0 26.1 "Functions of Opsins in Drosophila Taste". Current Biology 30 (8): 1367–1379.e6. April 2020. doi:10.1016/j.cub.2020.01.068. PMID 32243853.
- ↑ 27.0 27.1 27.2 27.3 27.4 "Shedding new light on opsin evolution". Proceedings. Biological Sciences 279 (1726): 3–14. January 2012. doi:10.1098/rspb.2011.1819. PMID 22012981.
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 28.6 28.7 "The Last Common Ancestor of Most Bilaterian Animals Possessed at Least Nine Opsins". Genome Biology and Evolution 8 (12): 3640–3652. December 2016. doi:10.1093/gbe/evw248. PMID 28172965.
- ↑ 29.0 29.1 "Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans". Cell 83 (2): 207–218. October 1995. doi:10.1016/0092-8674(95)90162-0. PMID 7585938.
- ↑ "Opsin evolution in the Ambulacraria". Marine Genomics 24 (Pt 2): 177–183. December 2015. doi:10.1016/j.margen.2015.10.001. PMID 26472700. Bibcode: 2015MarGn..24..177D.
- ↑ "Function of rhodopsin in temperature discrimination in Drosophila". Science 331 (6022): 1333–1336. March 2011. doi:10.1126/science.1198904. PMID 21393546. Bibcode: 2011Sci...331.1333S.
- ↑ "Drosophila auditory organ genes and genetic hearing defects". Cell 150 (5): 1042–1054. August 2012. doi:10.1016/j.cell.2012.06.043. PMID 22939627.
- ↑ "Rethinking Opsins". Molecular Biology and Evolution 39 (3): msac033. March 2022. doi:10.1093/molbev/msac033. PMID 35143663.
- ↑ 34.0 34.1 "Unconventional Roles of Opsins". Annual Review of Cell and Developmental Biology 33 (1): 241–264. October 2017. doi:10.1146/annurev-cellbio-100616-060432. PMID 28598695.
- ↑ 35.0 35.1 "Relocating the active-site lysine in rhodopsin and implications for evolution of retinylidene proteins". Proceedings of the National Academy of Sciences of the United States of America 110 (33): 13351–13355. August 2013. doi:10.1073/pnas.1306826110. PMID 23904486. Bibcode: 2013PNAS..11013351D.
- ↑ "Dissecting the conserved NPxxY motif of the M3 muscarinic acetylcholine receptor: critical role of Asp-7.49 for receptor signaling and multiprotein complex formation". Cellular Physiology and Biochemistry 28 (5): 1009–1022. 2011. doi:10.1159/000335788. PMID 22178951.
- ↑ 37.0 37.1 "Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation". Proceedings of the National Academy of Sciences of the United States of America 100 (5): 2290–2295. March 2003. doi:10.1073/pnas.0435715100. PMID 12601165. Bibcode: 2003PNAS..100.2290F.
- ↑ "Common activation mechanism of class A GPCRs". eLife 8: e50279. December 2019. doi:10.7554/eLife.50279. PMID 31855179.
- ↑ "Functional role of proline and tryptophan residues highly conserved among G protein-coupled receptors studied by mutational analysis of the m3 muscarinic receptor". The EMBO Journal 12 (1): 331–338. January 1993. doi:10.1002/j.1460-2075.1993.tb05661.x. PMID 7679072.
- ↑ "Mutation of Asn-391 within the conserved NPXXY motif of the cholecystokinin B receptor abolishes Gq protein activation without affecting its association with the receptor". The Journal of Biological Chemistry 275 (23): 17321–17327. June 2000. doi:10.1074/jbc.M909801199. PMID 10748160.
- ↑ 41.0 41.1 "The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium". The Journal of Biological Chemistry 274 (10): 6085–6090. March 1999. doi:10.1074/jbc.274.10.6085. PMID 10037690.
- ↑ "Rhodopsin and retinochrome in the squid retina". Nature 214 (5088): 573–575. May 1967. doi:10.1038/214573a0. PMID 6036171. Bibcode: 1967Natur.214..573H.
- ↑ 43.0 43.1 "Diversity and functional properties of bistable pigments". Photochemical & Photobiological Sciences 9 (11): 1435–1443. November 2010. doi:10.1039/c0pp00168f. PMID 20852774.
- ↑ 44.00 44.01 44.02 44.03 44.04 44.05 44.06 44.07 44.08 44.09 44.10 44.11 "The opsins". Genome Biology 6 (3): 213. 1 March 2005. doi:10.1186/gb-2005-6-3-213. PMID 15774036.
- ↑ "Identification and characterization of a protostome homologue of peropsin from a jumping spider". Journal of Comparative Physiology A 196 (1): 51–59. January 2010. doi:10.1007/s00359-009-0493-9. PMID 19960196.
- ↑ "The evolution of vision". Wiley Interdisciplinary Reviews. Developmental Biology 3 (1): 1–40. January 2014. doi:10.1002/wdev.96. PMID 24902832.
- ↑ 47.0 47.1 47.2 "Two Opsin 3-Related Proteins in the Chicken Retina and Brain: A TMT-Type Opsin 3 Is a Blue-Light Sensor in Retinal Horizontal Cells, Hypothalamus, and Cerebellum". PLOS ONE 11 (11): e0163925. 18 November 2016. doi:10.1371/journal.pone.0163925. PMID 27861495. Bibcode: 2016PLoSO..1163925K.
- ↑ "The role of proline residues in the structure and function of human MT2 melatonin receptor". Journal of Pineal Research 45 (4): 361–372. November 2008. doi:10.1111/j.1600-079X.2008.00598.x. PMID 18544139.
- ↑ "Molecular evolution of vertebrate visual pigments". Progress in Retinal and Eye Research 19 (4): 385–419. July 2000. doi:10.1016/S1350-9462(00)00002-1. PMID 10785616.
- ↑ "The molecular basis of variation in human color vision". Clinical Genetics 67 (5): 369–377. May 2005. doi:10.1111/j.1399-0004.2004.00343.x. PMID 15811001.
- ↑ 51.0 51.1 51.2 51.3 51.4 51.5 "Opsin expression in human epidermal skin". Photochemistry and Photobiology 91 (1): 117–123. 2015. doi:10.1111/php.12354. PMID 25267311.
- ↑ "Identification of a novel asthma susceptibility gene on chromosome 1qter and its functional evaluation". Human Molecular Genetics 17 (13): 1890–1903. July 2008. doi:10.1093/hmg/ddn087. PMID 18344558.
- ↑ 53.0 53.1 53.2 53.3 "Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain". The Journal of Neuroscience 19 (10): 3681–3690. May 1999. doi:10.1523/JNEUROSCI.19-10-03681.1999. PMID 10234000.
- ↑ "Encephalopsin (OPN3) protein abundance in the adult mouse brain". Journal of Comparative Physiology A 198 (11): 833–839. November 2012. doi:10.1007/s00359-012-0754-x. PMID 22991144.
- ↑ 55.0 55.1 "Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades". Proceedings. Biological Sciences 280 (1759): 20122987. May 2013. doi:10.1098/rspb.2012.2987. PMID 23554393.
- ↑ 56.0 56.1 56.2 56.3 "UV-sensitive photoreceptor protein OPN5 in humans and mice". PLOS ONE 6 (10): e26388. 17 October 2011. doi:10.1371/journal.pone.0026388. PMID 22043319. Bibcode: 2011PLoSO...626388K.
- ↑ "Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue". FEBS Letters 554 (3): 410–416. November 2003. doi:10.1016/S0014-5793(03)01212-2. PMID 14623103.
- ↑ "Evolution of mammalian Opn5 as a specialized UV-absorbing pigment by a single amino acid mutation". The Journal of Biological Chemistry 289 (7): 3991–4000. February 2014. doi:10.1074/jbc.M113.514075. PMID 24403072.
- ↑ "Evidence for distributed light sensing in the skin of cuttlefish, Sepia officinalis". Biology Letters 6 (5): 600–603. October 2010. doi:10.1098/rsbl.2010.0223. PMID 20392722.
- ↑ "Octopuses, and Maybe Squid, Can Sense Light With Their Skin". National Geographic. 20 May 2015. https://www.nationalgeographic.com/science/article/octopuses-and-maybe-squid-can-sense-light-with-their-skin.
- ↑ "Adaptive optoelectronic camouflage systems with designs inspired by cephalopod skins". Proceedings of the National Academy of Sciences of the United States of America 111 (36): 12998–13003. September 2014. doi:10.1073/pnas.1410494111. PMID 25136094. Bibcode: 2014PNAS..11112998Y.
- ↑ 62.0 62.1 "Cubozoan genome illuminates functional diversification of opsins and photoreceptor evolution". Scientific Reports 5: 11885. July 2015. doi:10.1038/srep11885. PMID 26154478. Bibcode: 2015NatSR...511885L.
- ↑ "A gonad-expressed opsin mediates light-induced spawning in the jellyfish Clytia". eLife 7: e29555. January 2018. doi:10.7554/eLife.29555. PMID 29303477.
- ↑ 64.0 64.1 64.2 "Extraocular, rod-like photoreceptors in a flatworm express xenopsin photopigment". eLife 8: e45465. October 2019. doi:10.7554/eLife.45465. PMID 31635694.
- ↑ 65.0 65.1 "Metazoan opsin evolution reveals a simple route to animal vision". Proceedings of the National Academy of Sciences of the United States of America 109 (46): 18868–18872. November 2012. doi:10.1073/pnas.1204609109. PMID 23112152. Bibcode: 2012PNAS..10918868F.
- ↑ 66.0 66.1 66.2 66.3 66.4 66.5 "Evolution of opsins and phototransduction". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364 (1531): 2881–2895. October 2009. doi:10.1098/rstb.2009.0051. PMID 19720651.
- ↑ 67.0 67.1 67.2 67.3 "The evolution of phototransduction from an ancestral cyclic nucleotide gated pathway". Proceedings. Biological Sciences 277 (1690): 1963–1969. July 2010. doi:10.1098/rspb.2009.1797. PMID 20219739.
- ↑ "Evolution and spectral tuning of visual pigments in birds and mammals". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364 (1531): 2941–2955. October 2009. doi:10.1098/rstb.2009.0044. PMID 19720655.
- ↑ "Opsins: evolution in waiting". Current Biology 15 (19): R794–R796. October 2005. doi:10.1016/j.cub.2005.09.025. PMID 16213808.
- ↑ "Photocyclic behavior of rhodopsin induced by an atypical isomerization mechanism". Proceedings of the National Academy of Sciences of the United States of America 114 (13): E2608–E2615. March 2017. doi:10.1073/pnas.1617446114. PMID 28289214.
- ↑ "Exo-rhodopsin: a novel rhodopsin expressed in the zebrafish pineal gland". Brain Research. Molecular Brain Research 73 (1–2): 110–118. November 1999. doi:10.1016/S0169-328X(99)00242-9. PMID 10581404.
- ↑ "Adaptation of pineal expressed teleost exo-rod opsin to non-image forming photoreception through enhanced Meta II decay". Cellular and Molecular Life Sciences 68 (22): 3713–3723. November 2011. doi:10.1007/s00018-011-0665-y. PMID 21416149.
- ↑ "Pinopsin is a chicken pineal photoreceptive molecule". Nature 372 (6501): 94–97. November 1994. doi:10.1038/372094a0. PMID 7969427. Bibcode: 1994Natur.372...94O.
- ↑ "Photoperiodic Regulation of Reproduction in Vertebrates". Annual Review of Animal Biosciences (Annual Reviews) 7 (1): 173–194. February 2019. doi:10.1146/annurev-animal-020518-115216. PMID 30332291.
- ↑ "Vertebrate ancient (VA) opsin and extraretinal photoreception in the Atlantic salmon (Salmo salar)". The Journal of Experimental Biology 203 (Pt 12): 1925–1936. June 2000. doi:10.1242/jeb.203.12.1925. PMID 10821749.
- ↑ "Nonvisual Opsins and the Regulation of Peripheral Clocks by Light and Hormones". Photochemistry and Photobiology 91 (5): 1046–1055. 2015. doi:10.1111/php.12494. PMID 26174318.
- ↑ "Parapinopsin, a novel catfish opsin localized to the parapineal organ, defines a new gene family". The Journal of Neuroscience 17 (21): 8083–8092. November 1997. doi:10.1523/JNEUROSCI.17-21-08083.1997. PMID 9334384.
- ↑ "Bistable UV pigment in the lamprey pineal". Proceedings of the National Academy of Sciences of the United States of America 101 (17): 6687–6691. April 2004. doi:10.1073/pnas.0400819101. PMID 15096614. Bibcode: 2004PNAS..101.6687K.
- ↑ "Diversification of non-visual photopigment parapinopsin in spectral sensitivity for diverse pineal functions". BMC Biology 13 (1): 73. September 2015. doi:10.1186/s12915-015-0174-9. PMID 26370232.
- ↑ "Parietal-eye phototransduction components and their potential evolutionary implications". Science 311 (5767): 1617–1621. March 2006. doi:10.1126/science.1123802. PMID 16543463. Bibcode: 2006Sci...311.1617S.
- ↑ "Diversity of animal opsin-based pigments and their optogenetic potential". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1837 (5): 710–716. May 2014. doi:10.1016/j.bbabio.2013.09.003. PMID 24041647.
- ↑ 82.0 82.1 82.2 "Characterization of a novel human opsin gene with wide tissue expression and identification of embedded and flanking genes on chromosome 1q43". Genomics 72 (2): 203–208. March 2001. doi:10.1006/geno.2001.6469. PMID 11401433.
- ↑ "Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain". Science 306 (5697): 869–871. October 2004. doi:10.1126/science.1099955. PMID 15514158. Bibcode: 2004Sci...306..869A.
- ↑ "A ciliary opsin in the brain of a marine annelid zooplankton is ultraviolet-sensitive, and the sensitivity is tuned by a single amino acid residue". The Journal of Biological Chemistry 292 (31): 12971–12980. August 2017. doi:10.1074/jbc.M117.793539. PMID 28623234.
- ↑ "A Go-type opsin mediates the shadow reflex in the annelid Platynereis dumerilii". BMC Biology 16 (1): 41. April 2018. doi:10.1186/s12915-018-0505-8. PMID 29669554.
- ↑ "Ciliary and rhabdomeric photoreceptor-cell circuits form a spectral depth gauge in marine zooplankton". eLife 7. May 2018. doi:10.7554/eLife.36440. PMID 29809157.
- ↑ 87.0 87.1 "Spectral Tuning of Phototaxis by a Go-Opsin in the Rhabdomeric Eyes of Platynereis". Current Biology 25 (17): 2265–2271. August 2015. doi:10.1016/j.cub.2015.07.017. PMID 26255845.
- ↑ "The evolution of eyes and visually guided behaviour". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364 (1531): 2833–2847. October 2009. doi:10.1098/rstb.2009.0083. PMID 19720648.
- ↑ "Eye evolution and its functional basis". Visual Neuroscience 30 (1–2): 5–20. March 2013. doi:10.1017/S0952523813000035. PMID 23578808.
- ↑ "Homologs of vertebrate Opn3 potentially serve as a light sensor in nonphotoreceptive tissue". Proceedings of the National Academy of Sciences of the United States of America 110 (13): 4998–5003. March 2013. doi:10.1073/pnas.1219416110. PMID 23479626. Bibcode: 2013PNAS..110.4998K.
- ↑ 91.0 91.1 "Diversity of Active States in TMT Opsins". PLOS ONE 10 (10): e0141238. 22 October 2015. doi:10.1371/journal.pone.0141238. PMID 26491964. Bibcode: 2015PLoSO..1041238S.
- ↑ 92.0 92.1 "Co-expression of VAL- and TMT-opsins uncovers ancient photosensory interneurons and motorneurons in the vertebrate brain". PLOS Biology 11 (6): e1001585. 11 June 2013. doi:10.1371/journal.pbio.1001585. PMID 23776409.
- ↑ 93.0 93.1 "Teleost multiple tissue (tmt) opsin: a candidate photopigment regulating the peripheral clocks of zebrafish?". Brain Research. Molecular Brain Research 112 (1–2): 135–145. April 2003. doi:10.1016/S0169-328X(03)00059-7. PMID 12670711.
- ↑ "Jellyfish vision starts with cAMP signaling mediated by opsin-G(s) cascade". Proceedings of the National Academy of Sciences of the United States of America 105 (40): 15576–15580. October 2008. doi:10.1073/pnas.0806215105. PMID 18832159. Bibcode: 2008PNAS..10515576K.
- ↑ "Evidence for multiple phototransduction pathways in a reef-building coral". PLOS ONE 7 (12): e50371. 5 December 2012. doi:10.1371/journal.pone.0050371. PMID 23227169. Bibcode: 2012PLoSO...750371M.
- ↑ "Evolution and functional diversity of jellyfish opsins". Current Biology 18 (1): 51–55. January 2008. doi:10.1016/j.cub.2007.11.059. PMID 18160295.
- ↑ "The comb jelly opsins and the origins of animal phototransduction". Genome Biology and Evolution 6 (8): 1964–1971. July 2014. doi:10.1093/gbe/evu154. PMID 25062921.
- ↑ "Opsins from the lateral eyes and ocelli of the horseshoe crab, Limulus polyphemus". Proceedings of the National Academy of Sciences of the United States of America 90 (13): 6150–6154. July 1993. doi:10.1073/pnas.90.13.6150. PMID 8327495. Bibcode: 1993PNAS...90.6150S.
- ↑ 99.0 99.1 "Opn5 is a UV-sensitive bistable pigment that couples with Gi and Gq subtype of G protein". Proceedings of the National Academy of Sciences of the United States of America 107 (51): 22084–22089. December 2010. doi:10.1073/pnas.1012498107. PMID 21135214. Bibcode: 2010PNAS..10722084Y.
- ↑ "Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea". Proceedings of the National Academy of Sciences of the United States of America 112 (42): 13093–13098. October 2015. doi:10.1073/pnas.1516259112. PMID 26392540. Bibcode: 2015PNAS..11213093B.
- ↑ "Analysis of the opsin repertoire in the tardigrade Hypsibius dujardini provides insights into the evolution of opsin genes in panarthropoda". Genome Biology and Evolution 6 (9): 2380–2391. September 2014. doi:10.1093/gbe/evu193. PMID 25193307.
- ↑ "A novel Go-mediated phototransduction cascade in scallop visual cells". The Journal of Biological Chemistry 272 (37): 22979–22982. September 1997. doi:10.1074/jbc.272.37.22979. PMID 9287291.
- ↑ "Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis- and all-trans-retinals as their chromophores". FEBS Letters 531 (3): 525–528. November 2002. doi:10.1016/s0014-5793(02)03616-5. PMID 12435605.
- ↑ "An opsin homologue in the retina and pigment epithelium". Investigative Ophthalmology & Visual Science 34 (13): 3669–3678. December 1993. PMID 8258527.
- ↑ "Molecular Evolution and Functional Diversity of Opsin-Based Photopigments". 20 October 2010. http://photobiology.info/Terakita.html.
- ↑ "The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light". The Journal of Biological Chemistry 280 (33): 29874–29884. August 2005. doi:10.1074/jbc.M503603200. PMID 15961402.
- ↑ "Retinal pigment epithelium-retinal G protein receptor-opsin mediates light-dependent translocation of all-trans-retinyl esters for synthesis of visual chromophore in retinal pigment epithelial cells". The Journal of Biological Chemistry 283 (28): 19730–19738. July 2008. doi:10.1074/jbc.M801288200. PMID 18474598.
- ↑ "Blue and ultraviolet light-absorbing opsin from the retinal pigment epithelium". Biochemistry 35 (20): 6251–6256. May 1996. doi:10.1021/bi952420k. PMID 8639565.
- ↑ "Peropsin, a novel visual pigment-like protein located in the apical microvilli of the retinal pigment epithelium". Proceedings of the National Academy of Sciences of the United States of America 94 (18): 9893–9898. September 1997. doi:10.1073/pnas.94.18.9893. PMID 9275222. Bibcode: 1997PNAS...94.9893S.
- ↑ Photobiology: The Science of Light and Life. Springer. 2 January 2015. p. 169. ISBN 978-1-4939-1468-5. https://books.google.com/books?id=VDgLBgAAQBAJ&pg=PA169. Retrieved 3 September 2015.
- ↑ "Casting a genetic light on the evolution of eyes". Science 313 (5795): 1914–1918. September 2006. doi:10.1126/science.1127889. PMID 17008522. Bibcode: 2006Sci...313.1914F.
- ↑ "Leptosphaeria rhodopsin: bacteriorhodopsin-like proton pump from a eukaryote". Proceedings of the National Academy of Sciences of the United States of America 102 (19): 6879–6883. May 2005. doi:10.1073/pnas.0409659102. PMID 15860584. Bibcode: 2005PNAS..102.6879W.
- ↑ "The opsin family of proteins". The Biochemical Journal 238 (3): 625–642. September 1986. doi:10.1042/bj2380625. PMID 2948499.
- ↑ "The evolutionary relationship between microbial rhodopsins and metazoan rhodopsins". TheScientificWorldJournal 2013: 435651. 2013. doi:10.1155/2013/435651. PMID 23476135.
- ↑ "Systematic study on G-protein couple receptor prototypes: did they really evolve from prokaryotic genes?". IET Systems Biology 8 (4): 154–161. August 2014. doi:10.1049/iet-syb.2013.0037. PMID 25075528.
- ↑ "Independent HHsearch, Needleman--Wunsch-based, and motif analyses reveal the overall hierarchy for most of the G protein-coupled receptor families". Molecular Biology and Evolution 28 (9): 2471–2480. September 2011. doi:10.1093/molbev/msr061. PMID 21402729.
- ↑ "The transporter-opsin-G protein-coupled receptor (TOG) superfamily". The FEBS Journal 280 (22): 5780–5800. November 2013. doi:10.1111/febs.12499. PMID 23981446.
- ↑ "Functional characterization of flavobacteria rhodopsins reveals a unique class of light-driven chloride pump in bacteria". Proceedings of the National Academy of Sciences of the United States of America 111 (18): 6732–6737. May 2014. doi:10.1073/pnas.1403051111. PMID 24706784. Bibcode: 2014PNAS..111.6732Y.
- ↑ "Of ion pumps, sensors and channels - perspectives on microbial rhodopsins between science and history". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1837 (5): 533–545. May 2014. doi:10.1016/j.bbabio.2013.08.006. PMID 23994288.
- ↑ "G protein-coupled time travel: evolutionary aspects of GPCR research". Molecular Interventions 7 (1): 17–25. February 2007. doi:10.1124/mi.7.1.5. PMID 17339603.
- ↑ "The microbial opsin family of optogenetic tools". Cell 147 (7): 1446–1457. December 2011. doi:10.1016/j.cell.2011.12.004. PMID 22196724.
 |



