Chemistry:Oritavancin
 | |
| Clinical data | |
|---|---|
| Pronunciation | /oʊˌrɪtəˈvænsɪn/ oh-RIT-ə-VAN-sin |
| Trade names | Orbactiv, Kimyrsa |
| Other names | LY333328 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614042 |
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 16 d)[4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C86H97Cl3N10O26 |
| Molar mass | 1793.12 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
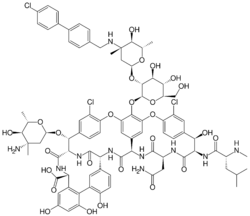
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin.[5]
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved oritavancin for treatment of acute bacterial skin and skin structure infections.[6][3]
Medical uses
Oritavancin is considered a long-lasting antibiotic due to its extended half-life (up to 16 d), high protein binding capacity, and ability to penetrate tissues effectively. It binds strongly to plasma proteins (around 85%), resulting in prolonged release into surrounding tissues. Furthermore, oritavancin exhibits excellent tissue penetration and distribution throughout various sites, including skin structures, synovial fluid (found in joints), bone tissue, and macrophages. Less frequent dosing requirements still keep efficacy against gram-positive infections, which is convenient for prolonged treatment courses such as osteoarticular infections and endocarditis, making it an option for outpatient antibiotic therapy in difficult-to-treat populations where adherence may be challenging and those with limited access to healthcare facilities.[4]
In vitro activity
Oritavancin shares certain properties with other members of the glycopeptide class of antibiotics, which includes vancomycin, the current standard of care for serious Gram-positive infections in the United States and Europe.[7] It possesses potent and rapid bactericidal activity in vitro against a broad spectrum of both resistant and susceptible Gram-positive bacteria, including Staphylococcus aureus, MRSA, enterococci, and streptococci.[8][9]
Oritavancin has potential use as a therapy for exposure to Bacillus anthracis, the Gram-positive bacterium that causes anthrax, having demonstrated efficacy in a mouse model both before and after exposure to the bacterium.[10] Oritavancin demonstrates in vitro activity against both the planktonic and biofilmstates of staphylococci associated with prosthetic joint infection (PJI), albeit with increased minimum biofilm bactericidal concentration (MBBC) compared to Minimum inhibitory concentrations (MIC) values.[11] Moreover oritavancin has demonstrated activity against in vitro to vancomycin-susceptible enterococci (VSE) and vancomycin-resistant enterococci (VRE) in both planktonic and biofilm states.[12]
Mechanism
The 4'-chlorobiphenylmethyl group disrupts the cell membrane of Gram-positive bacteria.[13] It also acts by inhibition of transglycosylation and inhibition of transpeptidation.[14]
Synergism
Several antibiotics have been tested as partner drugs of oritavancin.[15][16] Among these "companions" drugs, fosfomycin displayed (in vitro and in vivo) synergistic activity when administered together with oritavancin against VRE strains (both vanA and vanB), including biofilm-producing isolates.[17][18]
Spectrum of Activity
Oritavancin is active against gram-positive aerobic bacteria such as enterococci, staphylococci, streptococci, and anaerobic bacteria such as Clostridioides difficile , Clostridium perfringens , Peptostreptococcus spp. , and Propionibacterium acnes.[19][20] Oritavancin's spectrum of activity shows similarities to vancomycin, but with lower minimum inhibitory concentrations (MIC).[21]
Clinical trials
In 2003, results were presented from two pivotal phase-III clinical trials testing the efficacy of daily intravenous oritavancin for the treatment of acute bacterial skin and skin-structure infections (ABSSSI) caused by Gram-positive bacteria. The primary endpoints of both studies were met, with oritavancin achieving efficacy with fewer days of therapy than the comparator agents vancomycin followed by cephalexin. Oritavancin showed a statistically significant improved safety profile with a 19% relative reduction in the overall incidence of adverse events versus vancomycin/cephalexin in the second and larger pivotal trial.[22]
Osteomyelitis remains a formidable foe in an era of increasing incidence of Methicillin-resistant Staphylococcus aureus (MRSA) with limited guidance for treatment optimization. The success observed in many patients suggests multi-dose oritavancin may prove advantageous for chronic osteomyelitis but further research is needed to define the optimal dose and frequency of oritavancin for the treatment of chronic osteomyelitis.[23]
History
Originally discovered and developed by Eli Lilly, oritavancin was acquired by InterMune in 2001 and then by Targanta Therapeutics in late 2005.[24] In December 2008, the U.S. Food and Drug Administration (FDA) declined to approve oritavancin without additional studies, and an EU application was withdrawn.[citation needed]
In 2009, The Medicines Company acquired the development rights, completed clinical trials and submitted a new drug application to the FDA in February 2014.[25] On August 6, 2014, the United States FDA approved oritavancin to treat skin infections.[26]
A marketing authorisation valid throughout the European Union was granted on 19 March 2015, for the treatment of acute bacterial skin and skin structure infections in adults.[27]
References
- ↑ "Orbactiv- oritavancin injection, powder, lyophilized, for solution". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ff09a726-9f9b-4e30-b509-396781293220.
- ↑ "Kimyrsa- oritavancin diphosphate injection, powder, lyophilized, for solution". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5e755c40-6e73-4572-b474-4d8a131693d1.
- ↑ 3.0 3.1 "Orbactiv EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/orbactiv.
- ↑ 4.0 4.1 "The role of long-acting antibiotics in the clinical practice: a narrative review". Infez Med 31 (4): 449–465. 2023. doi:10.53854/liim-3104-4. PMID 38075413.
- ↑ "Interactions of oritavancin, a new lipoglycopeptide derived from vancomycin, with phospholipid bilayers: Effect on membrane permeability and nanoscale lipid membrane organization". Biochimica et Biophysica Acta (BBA) - Biomembranes 1788 (9): 1832–1840. September 2009. doi:10.1016/j.bbamem.2009.05.003. PMID 19450541.
- ↑ "FDA approves Orbactiv to treat skin infections" (Press release). U.S. Food and Drug Administration (FDA). 6 August 2014. Archived from the original on 8 August 2014.
- ↑ "A comparison of available and investigational antibiotics for complicated skin infections and treatment-resistant Staphylococcus aureus and enterococcus". Journal of Drugs in Dermatology 6 (1): 97–103. January 2007. PMID 17373167.
- ↑ "Oritavancin: a potential weapon in the battle against serious Gram-positive pathogens". Future Microbiology 3 (3): 251–263. June 2008. doi:10.2217/17460913.3.3.251. PMID 18505390.
- ↑ "Oritavancin: A New Lipoglycopeptide Antibiotic in the Treatment of Gram-Positive Infections". Infectious Diseases and Therapy 5 (1): 1–15. March 2016. doi:10.1007/s40121-016-0103-4. PMID 26831328.
- ↑ "Efficacy of oritavancin in a murine model of Bacillus anthracis spore inhalation anthrax". Antimicrobial Agents and Chemotherapy 52 (9): 3350–3357. September 2008. doi:10.1128/AAC.00360-08. PMID 18606841.
- ↑ "In vitro activity of oritavancin against biofilms of staphylococci isolated from prosthetic joint infection". Diagnostic Microbiology and Infectious Disease 92 (2): 155–157. October 2018. doi:10.1016/j.diagmicrobio.2018.05.010. PMID 29885758.
- ↑ "In vitro activity of oritavancin against planktonic and biofilm states of vancomycin-susceptible and vancomycin-resistant enterococci". Diagnostic Microbiology and Infectious Disease 91 (4): 348–350. August 2018. doi:10.1016/j.diagmicrobio.2018.03.008. PMID 29678300.
- ↑ "Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant enterococci to effect rapid bacterial killing". Antimicrobial Agents and Chemotherapy 54 (12): 5369–5371. December 2010. doi:10.1128/AAC.00760-10. PMID 20876372.
- ↑ "Oritavancin: mechanism of action". Clinical Infectious Diseases 54 (Suppl 3): S214–S219. April 2012. doi:10.1093/cid/cir920. PMID 22431851.
- ↑ "β-Lactam combinations with daptomycin provide synergy against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium". The Journal of Antimicrobial Chemotherapy 70 (6): 1738–1743. 2015-06-01. doi:10.1093/jac/dkv007. PMID 25645208.
- ↑ "In vitro activity of oritavancin alone or in combination against vancomycin-susceptible and -resistant enterococci". The Journal of Antimicrobial Chemotherapy 74 (5): 1300–1305. May 2019. doi:10.1093/jac/dkz010. PMID 30753495.
- ↑ "In Vitro and In Vivo Studies of Oritavancin and Fosfomycin Synergism against Vancomycin-Resistant Enterococcus faecium". Antibiotics 11 (10): 1334. September 2022. doi:10.3390/antibiotics11101334. PMID 36289992.
- ↑ "Vancomycin-resistant enterococcus bloodstream infection successfully managed with oritavancin and fosfomycin as sequential treatment". Journal of Chemotherapy: 1–4. August 2023. doi:10.1080/1120009X.2023.2247205. PMID 37602423.
- ↑ "Oritavancin activity against vancomycin-susceptible and vancomycin-resistant Enterococci with molecularly characterized glycopeptide resistance genes recovered from bacteremic patients, 2009-2010". Antimicrobial Agents and Chemotherapy 56 (3): 1639–1642. March 2012. doi:10.1128/AAC.06067-11. PMID 22183169.
- ↑ "Activity of oritavancin tested against uncommonly isolated Gram-positive pathogens responsible for documented infections in hospitals worldwide". The Journal of Antimicrobial Chemotherapy 69 (6): 1579–1581. June 2014. doi:10.1093/jac/dku016. PMID 24505091.
- ↑ "Comparative in vitro activity profile of oritavancin against recent gram-positive clinical isolates". Antimicrobial Agents and Chemotherapy 53 (11): 4762–4771. November 2009. doi:10.1128/AAC.00952-09. PMID 19738026.
- ↑ "Oritavancin for the treatment of acute bacterial skin and skin structure infections: an evidence-based review". Core Evidence 10: 39–47. 2015. doi:10.2147/CE.S51284. PMID 25709561.
- ↑ "Treatment of chronic osteomyelitis with multidose oritavancin: A case series and literature review". International Journal of Antimicrobial Agents 53 (4): 429–434. April 2019. doi:10.1016/j.ijantimicag.2018.11.023. PMID 30537532.
- ↑ Tomoko Okudaira (2014-05-09). "The Daily Biopharmaceutical News Source". BioWorld. http://www.bioworld.com/servlet/com.accumedia.web.Dispatcher?next=bioWorldHeadlines_article&forceid=46239.
- ↑ "Biotechs pick up slack in antibiotics development". 17 May 2011. http://www.fiercebiotech.com/story/biotechs-pick-slack-antibiotics-development/2011-05-17.
- ↑ "FDA approves Orbactiv to treat skin infections" (Press release). U.S. Food and Drug Administration (FDA). 6 August 2014. Archived from the original on 8 August 2014.
- ↑ "EPAR summary: Orbactiv". European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/003785/WC500186346.pdf.
External links
- "Oritavancin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/oritavancin.
 |

