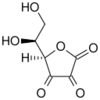
Chemistry:Dehydroascorbic acid
| |
| Names | |
|---|---|
| IUPAC name
L-threo-Hexo-2,3-diulosono-1,4-lactone
| |
| Systematic IUPAC name
(5R)-5-[(1S)-1,2-Dihydroxyethyl]oxolane-2,3,4-trione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H6O6 | |
| Molar mass | 174.108 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dehydroascorbic acid (DHA) is the major oxidized form of ascorbic acid (vitamin C). It is actively imported into the endoplasmic reticulum of cells via glucose transporters.[1] It is trapped therein by reduction back to ascorbic acid by glutathione and other thiols.[2] The (free) chemical radical semidehydroascorbic acid (SDA) also belongs to the group of oxidized ascorbic acids.
Structure and physiology
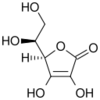
|

|
(reduced form of vitamin C)
Bottom: dehydroascorbic acid
(nominal oxidized form of vitamin C)
Although sodium-dependent transporters for vitamin C exists, it is present mainly in specialized cells whereas the glucose transporters, most notably GLUT1, transport DHA in most cells,[3] where recycling back to ascorbic acid generates the necessary enzyme cofactor and intracellular antioxidant, (see Transport to mitochondria).
The structure shown here for DHA is the commonly shown textbook structure. This 1,2,3-tricarbonyl is too electrophilic to survive more than a few milliseconds in aqueous solution, however. The actual structure shown by spectroscopic studies is the result of rapid hemiketal formation between the 6-OH and the 3-carbonyl groups. Hydration of the 2-carbonyl is also observed.[4] The lifetime of the stabilized species is commonly said to be about 6 minutes under biological conditions.[1] Destruction results from irreversible hydrolysis of the lactone bond, with additional degradation reactions following.[5] Crystallization of solutions of DHA gives a pentacyclic dimer structure of indefinite stability. Recycling of vitamin C via active transport of DHA into cells, followed by reduction and reuse, mitigates the inability of humans to synthesize it from glucose.[6]

Transport to mitochondria
Vitamin C accumulates in mitochondria, where most of the free radicals are produced, by entering as DHA through the glucose transporter GLUT10. Ascorbic acid protects the mitochondrial genome and membrane.[3]
Transport to the brain
Vitamin C does not pass from the bloodstream into the brain, although the brain is one of the organs that have the greatest concentration of vitamin C. Instead, DHA is transported through the blood–brain barrier via GLUT1 transporters, and then reduced back to ascorbic acid.[8]
Use
Dehydroascorbic acid has been used as a vitamin C dietary supplement.[9]
As a cosmetic ingredient, dehydroascorbic acid is used to enhance the appearance of the skin.[10] It may be used in a process for permanent waving of hair[11] and in a process for sunless tanning of skin.[12]
In a cell culture growth medium, dehydroascorbic acid has been used to assure the uptake of vitamin C into cell types that do not contain ascorbic acid transporters.[13]
As a pharmaceutical agent, some research has suggested that administration of dehydroascorbic acid may confer protection from neuronal injury following an ischemic stroke.[8] The literature contains many reports on the antiviral effects of vitamin C,[14] and one study suggests dehydroascorbic acid has stronger antiviral effects and a different mechanism of action than ascorbic acid.[15] Solutions in water containing ascorbic acid and copper ions and/or peroxide, resulting in rapid oxidation of ascorbic acid to dehydroascorbic acid, have been shown to possess powerful but short-lived antimicrobial, antifungal, and antiviral properties, and have been used to treat gingivitis, periodontal disease, and dental plaque.[16][17] A pharmaceutical product named Ascoxal is an example of such a solution used as a mouth rinse as an oral mucolytic and prophylactic agent against gingivitis.[17][18] Ascoxal solution has also been tested with positive results as a treatment for recurrent mucocutaneous herpes,[18] and as a mucolytic agent in acute and chronic pulmonary disease such as emphysema, bronchitis, and asthma by aerosol inhalation.[19]
References
- ↑ 1.0 1.1 May, J. M. (1998). "Ascorbate function and metabolism in the human erythrocyte". Frontiers in Bioscience 3 (4): d1–10. doi:10.2741/a262. PMID 9405334.
- ↑ Welch, R. W.; Wang, Y.; Crossman, A. Jr.; Park, J. B.; Kirk, K. L.; Levine, M. (1995). "Accumulation of Vitamin C (Ascorbate) and Its Oxidized Metabolite Dehydroascorbic Acid Occurs by Separate Mechanisms". Journal of Biological Chemistry 270 (21): 12584–12592. doi:10.1074/jbc.270.21.12584. PMID 7759506.
- ↑ 3.0 3.1 Lee, Y. C.; Huang, H. Y.; Chang, C. J.; Cheng, C. H.; Chen, Y. T. (2010). "Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: Mechanistic insight into arterial tortuosity syndrome". Human Molecular Genetics 19 (19): 3721–33. doi:10.1093/hmg/ddq286. PMID 20639396.
- ↑ Kerber, R. C. (2008). ""As Simple as Possible, but Not Simpler"—The Case of Dehydroascorbic Acid". Journal of Chemical Education 85 (9): 1237. doi:10.1021/ed085p1237. Bibcode: 2008JChEd..85.1237K.
- ↑ Kimoto, E.; Tanaka, H.; Ohmoto, T.; Choami, M. (1993). "Analysis of the transformation products of dehydro-L-ascorbic acid by ion-pairing high-performance liquid chromatography". Analytical Biochemistry 214 (1): 38–44. doi:10.1006/abio.1993.1453. PMID 8250252.
- ↑ Montel-Hagen, A.; Kinet, S.; Manel, N.; Mongellaz, C.; Prohaska, R.; Battini, J. L.; Delaunay, J.; Sitbon, M. et al. (2008). "Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C". Cell 132 (6): 1039–48. doi:10.1016/j.cell.2008.01.042. PMID 18358815.
- ↑ Koliou, Eleftheria K.; Ioannou, Panayiotis V. (February 2005). "Preparation of dehydro-l-ascorbic acid dimer by air oxidation of l-ascorbic acid in the presence of catalytic amounts of copper(II) acetate and pyridine" (in en). Carbohydrate Research 340 (2): 315–318. doi:10.1016/j.carres.2004.11.015. PMID 15639252. https://linkinghub.elsevier.com/retrieve/pii/S000862150400494X.
- ↑ 8.0 8.1 Huang, J.; Agus, D. B.; Winfree, C. J.; Kiss, S.; Mack, W. J.; McTaggart, R. A.; Choudhri, T. F.; Kim, L. J. et al. (2001). "Dehydroascorbic acid, a blood–brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke". Proceedings of the National Academy of Sciences of the United States of America 98 (20): 11720–11724. doi:10.1073/pnas.171325998. PMID 11573006. Bibcode: 2001PNAS...9811720H.
- ↑ Higdon, Jane (May 2001). "The Bioavailability of Different Forms of Vitamin C". The Linus Pauling Institute. http://lpi.oregonstate.edu/ss01/bioavailability.html.
- ↑ Kitt, D.Q. (2012), Topical Dehydroascorbic Acid (Oxidized Vitamin C) Permeates Stratum Corneum More Rapidly Than Ascorbic Acid, https://www.researchgate.net/publication/225274699, retrieved 2012-07-31
- ↑ US Patent 6,506,373 (issued January 14, 2003)
- ↑ U.S. Patent Application No. 10/685,073 Publication No. 20100221203 (published September 2, 2010)
- ↑ "Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs". Cancer Research 68 (19): 8031–8038. 2008. doi:10.1158/0008-5472.CAN-08-1490. PMID 18829561.
- ↑ Jariwalla, R.J. & Harakeh S. (1997). Mechanisms underlying the action of vitamin C in viral and immunodeficiency disease. In L. Packer & J. Fuchs (Eds.), Vitamin C in health and disease (pp. 309-322). New York:Marcell Dekker, Inc.
- ↑ Ericsson, Sten et al. "Anti Infectant Topical Preparations." U.S. Patent 3,065,139, filed November 9, 1954 and issued November 20, 1962
- ↑ 17.0 17.1 Fine, Daniel. "Gel composition for reduction of gingival inflammation and retardation of dental plaque." U.S Patent 5,298,237, filed Jan.24, 1992 and issued March 29, 1994
- ↑ 18.0 18.1 "Topical treatment of recurrent mucocutaneous herpes with ascorbic acid-containing solution". Antiviral Res 27 (3): 263–70. 1995. doi:10.1016/0166-3542(95)00010-j. PMID 8540748.
- ↑ "Clinical evaluation of Ascoxal: a new mucolytic agent". Anesthesia and Analgesia 45 (5): 531–534. 1966. doi:10.1213/00000539-196645050-00003. PMID 5330913.
Further reading
- "Recycling of vitamin C by a bystander effect". J Biol Chem 278 (12): 10128–33. 2003. doi:10.1074/jbc.M210686200. PMID 12435736.
External links
 |
