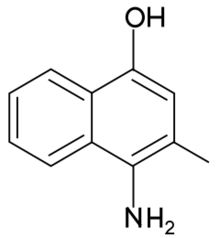
Chemistry:4-Amino-3-methyl-1-naphthol
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Amino-3-methylnaphthalen-1-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H11NO | |
| Molar mass | 173.215 g·mol−1 |
| Appearance | crystalline (HCl)[1] |
| Melting point | 270 °C [1] HCl decays |
| HCl is soluble[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-amino-3-methyl-1-naphthol is a synthetic menadione analog. It is also known as vitamin K7,[1] and was named as such in 1950 when it was recognized as a compound with vitamin K activity.[2][3]
It can be made from 2-methylnaphthalene or menadione. It forms a crystalline hydrochloride salt (C11H11NO·HCl) from hydrochloric acid. At least 1 g of the salt dissolves in 25 ml of water at 75 °C. The salt turns pink to dark violet on exposure to air and light.[1]
4-Amino-3-methyl-1-naphthol or its hydrochloride have not been used as commercial medicinal forms of vitamin K unlike phylloquinone and menadione for example.[4]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 The Merck index (12th ed.). Chapman & Hall Electronic Pub. Division. 2000. pp. 1581. ISBN 9781584881292.
- ↑ "Synthesis of 3-methyl-4-amino-1-naphthol hydrochloride (vitamin K7) and related vitamin-K-active compounds". Zeitschrift für Vitamin-, Hormon- und Fermentforschung 3 (3–4): 324–345. 1949–1950. ISSN 0373-0220. PMID 14782638.
- ↑ "Fat-soluble vitamins". Annual Review of Biochemistry 20 (1): 265–304. 1951. doi:10.1146/annurev.bi.20.070151.001405. PMID 14847531.
- ↑ "Anaphylactoid reactions to vitamin K". Journal of Thrombosis and Thrombolysis 11 (2): 175–183. 2001. doi:10.1023/A:1011237019082. ISSN 1573-742X. PMID 11406734.
 |

