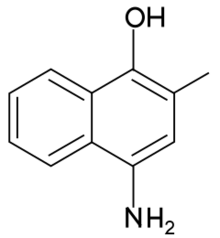
Chemistry:4-Amino-2-methyl-1-naphthol
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Amino-2-methylnaphthalen-1-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H11NO | |
| Molar mass | 173.215 g·mol−1 |
| Appearance | HCl: white crystalline powder[1] |
| Solubility | HCl: soluble in water, poorly soluble in ethanol, insoluble in diethyl ether[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-Amino-2-methyl-1-naphthol is a menadione analog. Its water-soluble hydrochloride (HCl) salt is often called vitamin K5. The HCl salt has been used as a medicine for vitamin K deficiency under tradenames such as Synkamin,[1][2] which was sold by Parke-Davis, but has since been discontinued.[3]
Vitamin K function of the compound was first noted in 1940.[4][5]
Oral lethal dose for the HCl salt in rats is 0.7 g/kg.[5]
Uses
4-Amino-2-methyl-1-naphthol HCl salt is a vitamin K and prevents bleeding caused by vitamin K deficiency when given via intravenous or intramuscular injections at doses of about 1–3 mg. HCl salt is water-soluble and its parenteral administration requires no emulsifiers[6][7] unlike fat-soluble phylloquinone for example, which is often in formulations with lecithin or glycocholic acid.[8] Parenterally given 1 mg/ml aqueous solutions and orally taken 4 mg tablets of the HCl salt have been available commercially.[1]
Chemistry
4-Amino-2-methyl-1-naphthol HCl salt has a mass of 209.57 g/mol.[1] It darkens at 262 °C and decays without melting at 280–282 °C.[2]
HCl salt breaks down in aqueous solutions via oxidation which is quite fast at neutral pH. First a pink and later a purple precipitant forms. The colored precipitant is (4-oxy-2-methylnaphtylimine)-2-methyl-1,4-naphthoquinone, which is a condensation reaction product of 4-amino-2-methyl-1-naphthol and menadione. Latter is formed via oxidation and deamination of 4-amino-2-methyl-1-naphthol.[9]
4-Amino-2-methyl-1-naphthol can be made from 2-methylnaphthalene or menadione.[2]
Research
4-Amino-2-methyl-1-naphthol HCl salt prevents the growth of different molds and bacteria. Thus it has been studied as potential food preservative.[10][9]
HCl salt has been studied as a potential treatment for cancer as it prevents glycolysis in cancer cells, which provides them energy for growth.[11]
References
- ↑ 1.0 1.1 1.2 1.3 The vitamins; chemistry, physiology, pathology, methods (2nd ed.). Academic Press. 1971. pp. 443, 497, 492. doi:10.1016/C2013-0-07583-4. ISBN 9780126337631.
- ↑ 2.0 2.1 2.2 2.3 The Merck index (12th ed.). Chapman & Hall Electronic Pub. Division. 2000. pp. 1581. ISBN 9781584881292.
- ↑ "Anaphylactoid reactions to vitamin K". Journal of Thrombosis and Thrombolysis 11 (2): 175–183. 2001. doi:10.1023/A:1011237019082. ISSN 1573-742X. PMID 11406734.
- ↑ "The vitamin K activity of 4-amino-2-methyl-1-naphthol and 4-amino-3-methyl-1-naphthol". Journal of Biological Chemistry 133 (1): 285–286. 1940. doi:10.1016/S0021-9258(18)73384-X. ISSN 0021-9258. http://www.jbc.org/content/133/1/285.
- ↑ 5.0 5.1 "Water soluble antihemorrhagic substances I: synthesis of 2-methyl-4-aminonaphthol-1 hydrochloride and of 2-methyl-1,4-diaminonaphthalene dihydrochloride, the water soluble synthetic vitamins K5 and K6". Recueil des Travaux Chimiques des Pays-Bas 62 (2): 75–84. 1943. doi:10.1002/recl.19430620203. ISSN 0165-0513.
- ↑ "Parenteral administration of a watersoluble compound with vitamin K activity: 4-amino-2-methyl-1-naphthol hydrochloride". Archives of Surgery 41 (5): 1244–1250. 1940. doi:10.1001/archsurg.1940.01210050204012. ISSN 0272-5533.
- ↑ "Studies on the minimal effective dose of a water-soluble vitamin K substitute in the prevention of hypoprothrombinemia in the newborn infant". The Journal of Pediatrics 24 (3): 259–269. 1944. doi:10.1016/S0022-3476(44)80102-0. ISSN 0022-3476. https://www.jpeds.com/article/S0022-3476(44)80102-0/abstract.
- ↑ "A new mixed micellar preparation for oral vitamin K prophylaxis: randomised controlled comparison with an intramuscular formulation in breast fed infants". Archives of Disease in Childhood 79 (4): 300–305. 1998. doi:10.1136/adc.79.4.300. ISSN 0003-9888. PMID 9875038.
- ↑ 9.0 9.1 "Non-stoichiometric sulfhydryl loss with vitamin K5". Biochemical and Biophysical Research Communications 23 (5): 660–665. 1966. doi:10.1016/0006-291X(66)90450-5. ISSN 0006-291X. PMID 5963891.
- ↑ "Vitamin K5 as a fungistatic agent". Applied Microbiology 13 (5): 660–662. 1965. doi:10.1128/AM.13.5.660-662.1965. ISSN 0003-6919. PMID 5867645.
- ↑ "Shikonin, vitamin K3 and vitamin K5 inhibit multiple glycolytic enzymes in MCF-7 cells". Oncology Letters 15 (5): 7423–7432. 2018. doi:10.3892/ol.2018.8251. ISSN 1792-1074. PMID 29725454.
 |

