Chemistry:Previtamin D3
From HandWiki
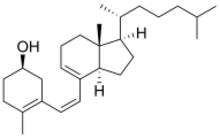
| |
| Names | |
|---|---|
| IUPAC name
(3S,6Z)-9,10-Secocholesta-5(10),6,8-trien-3-ol
| |
| Systematic IUPAC name
(1S)-4-Methyl-3-[(Z)-2-{(1R,3aR,7aR)-7a-methyl-1-[(2R)-6-methylheptan-2-yl]-2,3,3a,6,7,7a-hexahydro-1H-inden-4-yl}ethen-1-yl]cyclohex-3-en-1-ol | |
| Other names
Previtamin D3
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | Previtamin+D(3) |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H44O | |
| Molar mass | 384.648 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Previtamin D3 is an intermediate in the production of cholecalciferol (vitamin D3).
It is formed by the action of UV light, most specifically UVB light of wavelengths between 295 and 300 nm, acting on 7-dehydrocholesterol in the epidermal layers of the skin.[1][2][3]
The B ring of the steroid nucleus structure is broken open, making a secosteroid. This then undergoes spontaneous isomerization into cholecalciferol, the prohormone of the active form of vitamin D, calcitriol.
The synthesis of previtamin D3 is blocked effectively by sunscreens.[4]
Interactive pathway map
References
- ↑ "Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin". Science 216 (4549): 1001–3. May 1982. doi:10.1126/science.6281884. PMID 6281884. Bibcode: 1982Sci...216.1001M.
- ↑ "Who, what, where and when-influences on cutaneous vitamin D synthesis". Progress in Biophysics and Molecular Biology 92 (1): 17–25. September 2006. doi:10.1016/j.pbiomolbio.2006.02.004. PMID 16766240.
- ↑ "Action spectrum conversion factors that change erythemally weighted to previtamin D3-weighted UV doses". Photochemistry and Photobiology 84 (5): 1277–83. 2008. doi:10.1111/j.1751-1097.2008.00373.x. PMID 18513232. https://zenodo.org/record/848826.
- ↑ "Darkness at noon: sunscreens and vitamin D3". Photochemistry and Photobiology 83 (2): 459–63. 2007. doi:10.1562/2006-06-29-RC-956. PMID 17115796.
 |

