Chemistry:Doravirine
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Pifeltro |
| Other names | MK-1439 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618048 |
| License data |
|
| Routes of administration | By mouth[1][2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
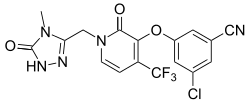
| Formula | C17H11ClF3N5O3 |
| Molar mass | 425.75 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Doravirine, sold under the brand name Pifeltro, is a non-nucleoside reverse transcriptase inhibitor medication developed by Merck & Co. for use in the treatment of HIV/AIDS.
Doravirine was approved for medical use in the United States in August 2018.[6]
References
- ↑ 1.0 1.1 "Pifeltro- doravirine tablet, film coated". 10 October 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=76ce1f00-28c0-4314-bd2c-d473fe3d0970.
- ↑ "The Antiretroviral Pipeline.". p. 10. http://www.pipelinereport.org/sites/g/files/g575521/f/201509/ARV.pdf.
- ↑ "PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION : PIFELTRO" (PDF). https://pdf.hres.ca/dpd_pm/00054609.PDF.
- ↑ "Pifeltro 100 mg film-coated tablets - Summary of Product Characteristics (SmPC)". https://www.medicines.org.uk/emc/product/9693/smpc.
- ↑ "Pifeltro EPAR". https://www.ema.europa.eu/en/medicines/human/EPAR/pifeltro.
- ↑ "Drug Approval Package: Pifeltro (doravirine)". 9 October 2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210806Orig1s000,210807Orig1s000TOC.cfm.
External links
- "Doravirine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/doravirine.
 |

