Chemistry:Atazanavir
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌætəˈzænəvɪər/ AT-ə-ZAN-ə-veer[1] |
| Trade names | Reyataz, Evotaz, others[2] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603019 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60-68% |
| Protein binding | 86% |
| Metabolism | Liver (CYP3A4-mediated) |
| Elimination half-life | 6.5 hours |
| Excretion | Fecal and kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
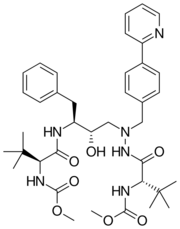
| Formula | C38H52N6O7 |
| Molar mass | 704.869 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Atazanavir, sold under the brand name Reyataz among others, is an antiretroviral medication used to treat HIV/AIDS.[2] It is generally recommended for use with other antiretrovirals.[2] It may be used for prevention after a needlestick injury or other potential exposure (postexposure prophylaxis (PEP)).[2] It is taken by mouth.[2]
Common side effects include headache, nausea, yellowish skin, abdominal pain, trouble sleeping, and fever.[2] Severe side effects include rashes such as erythema multiforme and high blood sugar.[2] Atazanavir appears to be safe to use during pregnancy.[2] It is of the protease inhibitor (PI) class and works by blocking HIV protease.[2]
Atazanavir was approved for medical use in the United States in 2003.[2] It is on the World Health Organization's List of Essential Medicines.[4] As of 2017 there is a generic version available in the United States manufactured by Teva Pharmaceuticals[5]
Medical uses
Atazanavir is used in the treatment of HIV. The efficacy of atazanavir has been assessed in a number of well-designed trials in ART-naive and ART-experienced adults.[6]
Atazanavir is distinguished from other protease inhibitors in that it has lesser effects on lipid profile and appears to be less likely to cause lipodystrophy. There may be some cross-resistant with other protease inhibitors.[2] When boosted with ritonavir it is equivalent in potency to lopinavir for use in salvage therapy in people with a degree of drug resistance, although boosting with ritonavir reduces the metabolic advantages of atazanavir.
Pregnancy
No evidence of harm has been found among pregnant women taking atazanavir. It is one of the preferred HIV medications to use in pregnant women who have not taken an HIV medication before.[7] It was not associated with any birth defects among over 2,500 live births observed. Atazanavir resulted in a better cholesterol profile and confirmed that it is a safe option during pregnancy.[7]
Contraindications
Atazanavir is contraindicated in those with previous hypersensitivity (e.g., Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions). Additionally, atazanavir should not be given with alfuzosin, rifampin, irinotecan, lurasidone, pimozide, triazolam, orally administered midazolam, ergot derivatives, cisapride, St. John's wort, lovastatin, simvastatin, sildenafil, indinavir, or nevirapine.[8]
Atazanavir inhibits the enzyme UDP glucuronosyltransferase (UGT) 1A1, thereby impacting the hepatic glucuronidation and elimination of bilirubin.[9] As such atazanavir may not be prescribed to patients with UGT1A1 deficiencies (e.g. those who suffer from Gilbert's syndrome or Crigler–Najjar syndrome) in order to avoid the possibility of jaundice.[9]
Adverse effects
Common side effects include: nausea, jaundice, rash, headache, abdominal pain, vomiting, insomnia, peripheral neurologic symptoms, dizziness, muscle pain, diarrhea, depression and fever.[8] Bilirubin levels in the blood are normally asymptomatically raised with atazanavir, but can sometimes lead to jaundice.[citation needed]
Mechanism of action
Atazanavir binds to the active site HIV protease and prevents it from cleaving the pro-form of viral proteins into the working machinery of the virus.[10] If the HIV protease enzyme does not work, the virus is not infectious, and no mature virions are made.[11][12] The azapeptide drug was designed as an analog of the peptide chain substrate that HIV protease would cleave normally into active viral proteins. More specifically, atazanavir is a structural analog of the transition state during which the bond between a phenylalanine and proline is broken.[13][14] Humans do not have any enzymes that break bonds between phenylalanine and proline, so this drug will not target human enzymes.[citation needed]
References
- ↑ "Atazanavir". MedlinePlus. National Institutes of Health. 15 October 2012. https://www.nlm.nih.gov/medlineplus/druginfo/meds/a603019.html.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 "Atazanavir Sulfate". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/atazanavir-sulfate.html.
- ↑ "Atazanavir (Reyataz) Use During Pregnancy". 27 February 2020. https://www.drugs.com/pregnancy/atazanavir.html.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Teva Announces Exclusive Launch of a Generic Version of Reyataz in the United States" (Press release). Teva. 27 December 2017. Retrieved 29 September 2021 – via Business Wire.
- ↑ 7.0 7.1 "What's New in the Guidelines? | Adult and Adolescent ARV Guidelines". https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0/.
- ↑ 8.0 8.1 "Reyataz Package Insert". Food and Drug Administration. September 2016. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021567s039,206352s004lbl.pdf.
- ↑ 9.0 9.1 "Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing". Clinical Pharmacology and Therapeutics 99 (4): 363–369. April 2016. doi:10.1002/cpt.269. PMID 26417955.
- ↑ "Atazanavir". DrugBank. 9 November 2016. https://www.drugbank.ca/drugs/DB01072.
- ↑ "Active human immunodeficiency virus protease is required for viral infectivity". Proceedings of the National Academy of Sciences of the United States of America 85 (13): 4686–4690. July 1988. doi:10.1073/pnas.85.13.4686. PMID 3290901. Bibcode: 1988PNAS...85.4686K.
- ↑ "HIV protease inhibitors: a review of molecular selectivity and toxicity". HIV/AIDS 7: 95–104. 2015. doi:10.2147/HIV.S79956. PMID 25897264.
- ↑ "HIV protease inhibitors". UpToDate. 17 June 2014.
- ↑ "New aza-dipeptide analogues as potent and orally absorbed HIV-1 protease inhibitors: candidates for clinical development". Journal of Medicinal Chemistry 41 (18): 3387–3401. August 1998. doi:10.1021/jm970873c. PMID 9719591.
 |
