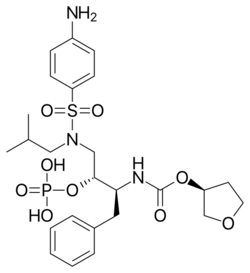
Chemistry:Fosamprenavir
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lexiva, Telzir |
| Other names | Fosamprenavir calcium (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604012 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | 90% |
| Metabolism | Hydrolysed to amprenavir and phosphate in GI tract epithelium |
| Elimination half-life | 7.7 hours |
| Excretion | Fecal (as metabolites of amprenavir) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL |
|
| NIAID ChemDB | |
| Chemical and physical data | |
| Formula | C25H36N3O9PS |
| Molar mass | 585.61 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fosamprenavir (FPV), sold under the brand names Lexiva and Telzir, is a medication used to treat HIV/AIDS.[3][4] It is a prodrug of the protease inhibitor and antiretroviral drug amprenavir.[5] It is marketed by ViiV Healthcare as the calcium salt.[3][4]
Fosamprenavir was approved for medical use in the United States in October 2003,[6] and in the European Union in July 2004.[4] The human body metabolizes fosamprenavir in order to form amprenavir, which is the active ingredient.[3]
A head-to-head study with lopinavir showed the two drugs to have comparable potency, but patients on fosamprenavir tended to have a higher serum cholesterol.[7]
Medical uses
Fosamprenavir is used for the treatment of HIV-1 infections, typically but not necessarily in combination with low-dose ritonavir or other antiviral drugs.[8][9]
Adverse effects
The most common adverse effect is diarrhea. Other common side effects include headache, dizziness and exanthema, which is usually transient. Severe allergic reactions (Stevens–Johnson syndrome) are rare.[8]
Interactions
Amprenavir (the active metabolite of fosamrenavir, which is found in blood plasma, liver and other organs) is metabolized via the liver enzyme CYP3A4 and also weakly inhibits this enzyme. This means that combination with drugs that are also metabolized by CYP3A4 can increase their plasma concentrations and thus side effects; and combination with drugs that inhibit CYP3A4 can increase amprenavir concentrations.[8]
When combining fosamprenavir with low doses of the CYP3A4 inhibitor ritonavir, this interaction is intended as it allows for application of lower fosamprenavir doses.[8]
Pharmacology
Fosamprenavir is quickly activated to amprenavir, even before it reaches the circulation. Amprenavir is a HIV protease inhibitor.[8]

References
- ↑ "Telzir Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=74692.
- ↑ "Telzir 700 mg film-coated tablets - Summary of Product Characteristics (SmPC)". 22 June 2021. https://www.medicines.org.uk/emc/product/5555.
- ↑ 3.0 3.1 3.2 3.3 "Lexiva- fosamprenavir calcium tablet, film coated Lexiva- fosamprenavir calcium suspension". 1 October 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24feb9be-32a6-45fd-a896-f3e202edd8a9.
- ↑ 4.0 4.1 4.2 4.3 "Telzir EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/telzir.
- ↑ "Fosamprenavir Monograph for Professionals". https://www.drugs.com/monograph/fosamprenavir.html.
- ↑ "Drug Approval Package: Lexiva (Fosamprenavir Calcium) NDA #021548". https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-548_Lexiva.cfm.
- ↑ "The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: a randomised non-inferiority trial". Lancet 368 (9534): 476–82. August 2006. doi:10.1016/S0140-6736(06)69155-1. PMID 16890834.
- ↑ 8.0 8.1 8.2 8.3 8.4 (in German) Austria-Codex (62nd ed.). Vienna: Österreichischer Apothekerverlag. 2007. pp. 8009–17. ISBN 978-3-85200-181-4.
- ↑ "Lexiva". Drugs.com. Monograph.
- ↑ "Amprenavir complexes with HIV-1 protease and its drug-resistant mutants altering hydrophobic clusters". The FEBS Journal 277 (18): 3699–714. September 2010. doi:10.1111/j.1742-4658.2010.07771.x. PMID 20695887.
External links
- "Fosamprenavir". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/fosamprenavir.
- "Fosamprenavir calcium". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/fosamprenavir%20calcium.
 |
