Chemistry:Elvitegravir
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Vitekta; Stribild (fixed-dose combination) |
| Other names | GS-9137 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 98% |
| Metabolism | liver, via CYP3A |
| Elimination half-life | 12.9 (8.7–13.7) hours |
| Excretion | liver 93%, renal 7% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
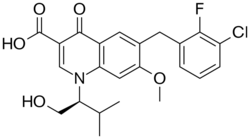
| Formula | C23H23ClFNO5 |
| Molar mass | 447.89 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Elvitegravir (EVG) is an integrase inhibitor used to treat HIV infection. It was developed[1] by the pharmaceutical company Gilead Sciences, which licensed EVG from Japan Tobacco in March 2008.[2][3][4] The drug gained approval by the U.S. Food and Drug Administration on August 27, 2012, for use in adult patients starting HIV treatment for the first time as part of the fixed dose combination known as Stribild.[5] On September 24, 2014, the FDA approved Elvitegravir as a single pill formulation under the trade name Vitekta.[6] On November 5, 2015, the FDA approved the drug for use in patients affected with HIV-1 as a part of a second fixed dose combination pill known as Genvoya.[7]
According to the results of the phase II clinical trial, patients taking once-daily elvitegravir boosted by ritonavir had greater reductions in viral load after 24 weeks compared to individuals randomized to receive a ritonavir-boosted protease inhibitor.[8]
Medical uses
In the United States, elvitegravir can be obtained either as part of the combination pills Stribild or Genvoya, or as the single pill formulation Vitekta.[9]
Vitekta is FDA approved to be used for the treatment of HIV-1 infection in adults who have previous treatment experience with antiretroviral therapy. It must be used in combination with a protease inhibitor that is coadministered with ritonavir as well as additional antiretroviral drug(s).[10]
Adverse effects
The most common side effects of taking elvitegravir are diarrhea (in 7% of patients) and nausea (4%). Other side effects that occurred in more than 1% of people are headache, tiredness, rashes, and vomiting.[10][11]
Interactions and contraindications
Elvitegravir is metabolised via the liver enzyme CYP3A. Substances that induce this enzyme can reduce elvitegravir concentrations in the body, potentially triggering the development of resistant virus strains. Consequently, co-administration of strong CYP3A inducers is contraindicated; examples are rifampicin, the anticonvulsants carbamazepine, phenobarbital and phenytoin, as well as St John's wort.[11]
Glucuronidation of elvitegravir is facilitated by the enzymes UGT1A1 and 3, resulting in increased blood plasma levels when taken together with strong UGT1A inhibitors such as ritonavir and other HIV protease inhibitors.[11][12] (But ritonavir also increases elvitegravir levels by inhibiting CYP3A.)
Furthermore, elvitegravir is a weak to medium inducer of CYP1A2, CYP2C19, CYP2C9, CYP3A, and a number of UGTs; the clinical relevance of these findings is however unclear.[11]
Pharmacology
Mechanism of action
Elvitegravir inhibits the enzyme integrase of HIV-1, and of HIV-2 to a lesser extent. The virus needs this enzyme to integrate its genetic code into the host's DNA.[11]
Pharmacokinetics
The drug is taken by mouth. When taken together with ritonavir and a meal, it reaches highest blood plasma concentrations after four hours. Bioavailability is better with fatty meals. In the bloodstream, 98–99% of the substance are bound to plasma proteins. It is metabolized mainly by CYP3A oxidation, and secondly by UGT1A1 and 3 glucuronidation. Nearly 95% are excreted via the feces, and the rest via urine. Plasma half-life when combined with ritonavir is 8.7 to 13.7 hours.[11]
References
- ↑ "Phase III Clinical Trial of Elvitegravir". Gilead Press Release. 22 July 2008. http://www.gilead.com/pr_1177855.
- ↑ "Gilead and Japan Tobacco Sign Licensing Agreement for Novel HIV Integrase Inhibitor". Gilead Press Release. March 22, 2008. http://www.gilead.com/pr_687733.
- ↑ "Broad antiretroviral activity and resistance profile of the novel human immunodeficiency virus integrase inhibitor elvitegravir (JTK-303/GS-9137)". Journal of Virology 82 (2): 764–774. January 2008. doi:10.1128/JVI.01534-07. PMID 17977962.
- ↑ "Antiviral drugs in the treatment of AIDS: what is in the pipeline ?". European Journal of Medical Research 12 (9): 483–495. October 2007. PMID 17933730.
- ↑ "Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks". Lancet 379 (9835): 2439–2448. June 2012. doi:10.1016/S0140-6736(12)60917-9. PMID 22748591.
- ↑ "FDA Approval Bulletin". http://content.govdelivery.com/accounts/USFDA/bulletins/d162bb.
- ↑ "Press Announcements - FDA approves new treatment for HIV" (in en). https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm471300.htm.
- ↑ "ICAAC: Best response to elvitegravir seen when used with T-20 and other active agents". Aidsmap.com. 19 September 2007. https://www.aidsmap.com/news/sep-2007/icaac-best-response-elvitegravir-seen-when-used-t-20-and-other-active-agents.
- ↑ "FDA Approved Drug Listing". http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- ↑ 10.0 10.1 "Vitekta Package Insert". Foster City, CA: Gilead Sciences, Inc.. 2014. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=be87c3bc-97bb-49cb-8053-9b0c756a1965#section-1.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 (in de) Austria-Codex. Vienna: Österreichischer Apothekerverlag. 2015.
- ↑ "In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation". Drug Metabolism and Disposition 33 (11): 1729–1739. November 2005. doi:10.1124/dmd.105.005447. PMID 16118329.
External links
- "Elvitegravir". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/elvitegravir.
 |

