Chemistry:S-Adenosyl-L-homocysteine
From HandWiki
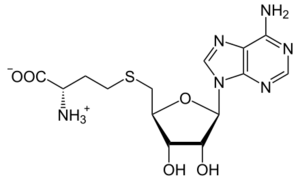
| |
| Names | |
|---|---|
| IUPAC name
S-(5′-Deoxyadenos-5′-yl)-L-homocysteine
| |
| Systematic IUPAC name
(2S)-2-Amino-4-({[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}sulfanyl)butanoic acid | |
| Other names
AdoHcy, 2-S-adenosyl-L-homocysteine,
5′-S-(3-Amino-3-carboxypropyl)-5′-thioadenosine S-adenosylhomocysteine, SAH | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| MeSH | S-Adenosylhomocysteine |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H20N6O5S | |
| Molar mass | 384.41 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
S-Adenosyl-L-homocysteine (SAH) is the biosynthetic precursor to homocysteine.[1] SAH is formed by the demethylation of S-adenosyl-L-methionine.[2][3] Adenosylhomocysteinase converts SAH into homocysteine and adenosine.
Biological role
DNA methyltransferases are inhibited by SAH.[4] Two S-adenosyl-L-homocysteine cofactor products can bind the active site of DNA methyltransferase 3B and prevent the DNA duplex from binding to the active site, which inhibits DNA methylation.[5]
References
- ↑ "Pathways and regulation of homocysteine metabolism in mammals". Seminars in Thrombosis and Hemostasis 26 (3): 219–225. 2000. doi:10.1055/s-2000-8466. PMID 11011839.
- ↑ "Biosynthesis of nitrogenase metalloclusters". Chemical Reviews 114 (8): 4063–4080. April 2014. doi:10.1021/cr400463x. PMID 24328215.
- ↑ "Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology". The Journal of Nutrition 132 (8 Suppl): 2361S–2366S. August 2002. doi:10.1093/jn/132.8.2361S. PMID 12163693.
- ↑ "Activation and inhibition of DNA methyltransferases by S-adenosyl-L-homocysteine analogues". Bioorganic & Medicinal Chemistry 16 (5): 2276–2285. March 2008. doi:10.1016/j.bmc.2007.11.075. PMID 18083524.
- ↑ "Structural insights into CpG-specific DNA methylation by human DNA methyltransferase 3B". Nucleic Acids Research 48 (7): 3949–3961. April 2020. doi:10.1093/nar/gkaa111. PMID 32083663.
External links
 |

