Chemistry:Lenacapavir
 | |
| Clinical data | |
|---|---|
| Trade names | Sunlenca |
| Other names | GS-CA1, GS-6207 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, subcutaneous |
| Drug class | Capsid inhibitors |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
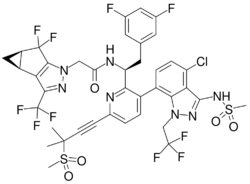
| Formula | C39H32ClF10N7O5S2 |
| Molar mass | 968.28 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lenacapavir, sold under the brand name Sunlenca, is an antiretroviral medication used to treat HIV/AIDS.[8] It is taken by mouth or by subcutaneous injection.[8]
The most common side effects include reactions at the injection site and nausea.[8][9]
Lenacapavir was approved for medical use in the European Union in August 2022,[8][10] in Canada in November 2022,[4][5] and in the United States in December 2022.[9][11][12] It is the first of a class of drugs called capsid inhibitors to be FDA-approved for treating HIV/AIDS.[9][13]
Medical uses
Lenacapavir, in combination with other antiretrovirals, is indicated for the treatment of HIV/AIDS. It is used in heavily treatment-experienced adults with multiple drug resistance in whom current antiretroviral therapy is ineffective due to resistance, intolerance or safety considerations.[7][9]
Mechanism of action
Lenacapavir works by binding directly to the interface between HIV-1 viral capsid protein (p24) subunits in capsid hexamers, interfering with essential steps of viral replication, including capsid-mediated nuclear uptake of HIV-1 proviral DNA, virus assembly and release, production of capsid protein subunits, and capsid core formation.[9][14] The US Food and Drug Administration considers it to be a first-in-class medication.[13][15]
History
Lenacapavir was developed by Gilead Sciences.[16]
As of 2021, it is in phase II/III clinical trials.[17] It is being investigated as a treatment for HIV patients infected with multidrug-resistant virus and as a twice-yearly injectable for pre-exposure prophylaxis.[17][18]
The safety and efficacy of lenacapavir were established through a multicenter clinical trial with 72 participants whose HIV infections were resistant to multiple classes of HIV medications.[9] These participants had to have high levels of virus in their blood despite being on antiretroviral drugs.[9] Participants were enrolled into one of two study groups.[9] One group was randomized to receive either lenacapavir or placebo in a double-blind fashion, and the other group received open-label lenacapavir.[9] The primary measure of efficacy was the proportion of participants in the randomized study group who achieved a certain level of reduction in virus during the initial 14 days compared to baseline.[9]
The U.S. Food and Drug Administration granted the application for lenacapavir priority review, fast track, and breakthrough therapy designations.[9]
Society and culture
Legal status
On 23 June 2022, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Sunlenca, intended for the treatment of adults with multidrug‑resistant human immunodeficiency virus type 1 (HIV‑1) infection.[19] The applicant for this medicinal product is Gilead Sciences Ireland UC.[19]
Lenacapavir was approved for medical use in the European Union in August 2022,[8][20] in Canada in November 2022,[4][5] and in the United States in December 2022.[9]
References
- ↑ 1.0 1.1 "Sunlenca". 6 April 2023. https://www.tga.gov.au/resources/auspmd/sunlenca.
- ↑ "Sunlenca lenacapavir (as sodium) 300 mg film coated tablet blister pack (392350)". 28 March 2023. https://www.tga.gov.au/resources/artg/392350.
- ↑ "Sunlenca lenacapavir (as sodium) 463.5 mg/1.5 mL solution for injection vial (386895)". 28 March 2023. https://www.tga.gov.au/resources/artg/386895.
- ↑ 4.0 4.1 4.2 "Sunlenca Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=102149.
- ↑ 5.0 5.1 5.2 "Sunlenca Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=102150.
- ↑ "Summary Basis of Decision - Sunlenca". 10 March 2023. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00627&lang=en.
- ↑ 7.0 7.1 "Sunlenca- lenacapavir sodium tablet, film coated Sunlenca- lenacapavir sodium kit". 21 December 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e5652804-29c4-40d7-aeb2-0142ed2a7b5b.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 "Sunlenca EPAR". 22 June 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/sunlenca. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 "FDA Approves New HIV Drug for Adults with Limited Treatment Options" (Press release). U.S. Food and Drug Administration (FDA). 22 December 2022. Retrieved 23 December 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Gilead Announces First Global Regulatory Approval of Sunlenca (Lenacapavir), the Only Twice-Yearly HIV Treatment Option". Gilead Sciences, Inc. (Press release). 22 August 2022. Retrieved 23 December 2022.
- ↑ "Sunlenca (lenacapavir) Receives FDA Approval as a First-in-Class, Twice-Yearly Treatment Option for People Living With Multi-Drug Resistant HIV". Gilead Sciences, Inc. (Press release). 22 December 2022. Retrieved 23 December 2022.
- ↑ "Lenacapavir: First Approval". Drugs 82 (14): 1499–1504. September 2022. doi:10.1007/s40265-022-01786-0. PMID 36272024.
- ↑ 13.0 13.1 "Advancing Health Through Innovation: New Drug Therapy Approvals 2022". 10 January 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Lenacapavir – Full prescribing information". December 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215973s000lbl.pdf.
- ↑ (PDF) New Drug Therapy Approvals 2022 (Report). January 2024. https://www.fda.gov/media/164429/download. Retrieved 14 January 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Clinical targeting of HIV capsid protein with a long-acting small molecule". Nature 584 (7822): 614–618. August 2020. doi:10.1038/s41586-020-2443-1. PMID 32612233. Bibcode: 2020Natur.584..614L.
- ↑ 17.0 17.1 "Lenacapavir Effective in Multidrug Resistant HIV". 11 March 2021. http://www.medscape.com/viewarticle/947319.
- ↑ "Lenacapavir Shows Promise for Long-Acting HIV Treatment and Prevention". 15 March 2021. https://www.poz.com/article/lenacapavir-shows-promise-longacting-hiv-treatment-prevention.
- ↑ 19.0 19.1 "Sunlenca: Pending EC decision". 23 June 2022. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/sunlenca. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Sunlenca Product information". https://ec.europa.eu/health/documents/community-register/html/h1671.htm.
External links
- "Lenacapavir". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/rn/2189684-44-2.
- "Lenacapavir sodium". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/lenacapavir%20sodium.
- "Lenacapavir". National Institutes of Health. https://clinicalinfo.hiv.gov/en/drugs/lenacapavir.
 |

