Chemistry:Pegcetacoplan
 | |
| Clinical data | |
|---|---|
| Trade names | Empaveli, Aspaveli, Syfovre |
| Other names | APL-2 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a621045 |
| License data | |
| Pregnancy category | |
| Routes of administration | Subcutaneous, intravitreal |
| Drug class | Complement inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
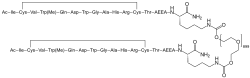
| Formula | C1970H3848N50O947S4 |
| Molar mass | 43520.10 g·mol−1 |
Pegcetacoplan, sold under the brand name Empaveli, among others, is a medication used to treat paroxysmal nocturnal hemoglobinuria[6][9][10][11][12] and geographic atrophy of the retina.[8][13] Pegcetacoplan is a complement inhibitor.[6][8]
The most common side effects include injection-site reactions, infections, diarrhea, abdominal pain, respiratory tract infection, viral infection, and fatigue.[6][9]
Paroxysmal nocturnal hemoglobinuria is characterized by red blood cell destruction, anemia (red blood cells unable to carry enough oxygen to tissues), blood clots, and impaired bone marrow function (not making enough blood cells).[7]
Pegcetacoplan is the first treatment for paroxysmal nocturnal hemoglobinuria that binds to and inhibits complement protein C3.[7] Pegcetacoplan was approved for medical use in the United States in May 2021.[7] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[14]
Medical uses
Pegcetacoplan is indicated to treat adults with paroxysmal nocturnal hemoglobinuria.[6][7][9] In February 2023, the indication was updated to include the treatment of people with geographic atrophy secondary to age-related macular degeneration.[8][15] The medication is given through a subcutaneous infusion for paroxysmal nocturnal hemoglobinuria and through intravitreal injection for age-related macular degeneration.[16]
Pharmacology
Pegcetacoplan acts as a complement inhibitor, specifically targeting complement protein C3, which plays a crucial role in the pathogenesis of paroxysmal nocturnal hemoglobinuria (PNH). In individuals with PNH, there is a heightened and uninhibited complement activity, which may lead to intravascular (inside blood vessels) or extravascular (within the liver or spleen) hemolysis.[6] By binding to and inhibiting C3, pegcetacoplan helps regulate complement activation, thereby reducing red blood cell destruction, anemia, blood clot formation, and improving bone marrow function. This targeted mechanism of action makes pegcetacoplan the first-in-class medication for the treatment of PNH, offering a promising therapeutic approach to address the underlying complement dysregulation in this condition.[17]
Pharmacokinetics
Pegcetacoplan exhibits proportional exposure with increasing doses and reaches peak concentration within 4.5–6 days after a single subcutaneous dose. Steady-state concentrations are achieved in about 4–6 weeks of treatment, with average serum trough concentrations ranging from 655-706 µg/mL. Pegcetacoplan is metabolized into smaller peptides and amino acids and has a median effective elimination half-life of approximately 8.0 days in patients with PNH.[16]
Adverse effects
Meningococcal (a type of bacteria) infections can occur in people taking pegcetacoplan.[7] Pegcetacoplan may also predispose individuals to serious infections, especially infections caused by encapsulated bacteria.[7] These infections include but are not limited to Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae.[6] Common adverse effects associated with the medication include stomach pain, vomiting, diarrhea, cold sores, common-cold like symptoms, tiredness as well as any itching, redness, or sensitivity at the injection site.[6] Pegcetacoplan may cause fetal harm.[6] Pegcetacoplan may also interfere with silica reagents in laboratory coagulation panels.[6]
History
The therapeutic efficacy of subcutaneous pegcetacoplan in treating paroxysmal nocturnal hemoglobinuria (PNH) has been established through several clinical trials. Initial phase I and II trials, such as PADDOCK, PALOMINO, and PHAROAH, evaluated pegcetacoplan in PNH patients who had not received a complement inhibitor or had previously received eculizumab. These trials demonstrated that 1–2 years of pegcetacoplan treatment effectively controlled hemolysis and improved quality of life in PNH patients.,[18][19]
Building upon these findings, the efficacy of pegcetacoplan was further assessed in phase III trials. The PRINCE trial, a 26-week study, focused on complement inhibitor-naïve patients with PNH, while the PEGASUS trial, a 48-week multinational study, included complement inhibitor-treated patients with PNH. In these trials, subcutaneous pegcetacoplan was administered at a dosage of 1080 mg twice weekly, delivered as a 20-mL subcutaneous infusion. Patients had the option to self-administer the medication or have it administered by qualified research personnel.[20]
Society and culture
Legal status
The FDA granted the application for pegcetacoplan orphan drug designation.[14]
On 14 October 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Aspaveli, intended for the treatment of adults with paroxysmal nocturnal hemoglobinuria.[21] The applicant for this medicinal product is Swedish Orphan Biovitrum AB (publ).[21] Pegcetacoplan was approved for the treatment of paroxysmal nocturnal haemoglobinuria in the European Union in December 2021.[9][22]
References
- ↑ Jump up to: 1.0 1.1 "Empaveli APMDS". Therapeutic Goods Administration (TGA). 17 February 2022. https://www.tga.gov.au/resources/auspmd/empaveli.
- ↑ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 21 December 2022. https://www.tga.gov.au/resources/resource/guidance/updates-prescribing-medicines-pregnancy-database.
- ↑ "Notice: Multiple Additions to the Prescription Drug List (PDL) [2023-03-08"]. 8 March 2023. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/prescription-drug-list/notices-changes/mutliple-additions-2023-03-08.html.
- ↑ "Summary Basis of Decision for Empaveli". 13 April 2023. https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1682951781384.
- ↑ "Aspaveli Summary of Product Characteristics (SmPC)". 21 September 2022. https://www.medicines.org.uk/emc/product/13369/smpc.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 6.9 "Empaveli- pegcetacoplan injection, solution". 13 April 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c23d89e9-b00b-4520-e053-2995a90a95af.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 7.5 7.6 "FDA approves new treatment for adults with serious rare blood disease". U.S. Food & Drug Administration (FDA). 18 May 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-new-treatment-adults-serious-rare-blood-disease.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 "Syfovre- pegcetacoplan injection, solution". 23 February 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b4434e61-9ec9-4e0e-bd60-45aaa0a87e64.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 "Aspaveli EPAR". European Medicines Agency (EMA). 6 September 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/aspaveli.
- ↑ "Pegcetacoplan: A Review in Paroxysmal Nocturnal Haemoglobinuria". Drugs 82 (18): 1727–1735. December 2022. doi:10.1007/s40265-022-01809-w. PMID 36459381.
- ↑ "Safety and efficacy of pegcetacoplan in paroxysmal nocturnal hemoglobinuria". Therapeutic Advances in Hematology (SAGE Publishing) 13: 20406207221114673. 28 July 2022. doi:10.1177/20406207221114673. PMID 35923770.
- ↑ "Novel targeted C3 inhibitor pegcetacoplan for paroxysmal nocturnal hemoglobinuria". Clinical and Experimental Medicine 23 (3): 717–726. July 2023. doi:10.1007/s10238-022-00830-3. PMID 35441351.
- ↑ "Pegcetacoplan treatment for geographic atrophy due to age-related macular degeneration: a plain language summary of the FILLY study". Immunotherapy 14 (13): 995–1006. September 2022. doi:10.2217/imt-2022-0078. PMID 35860926.
- ↑ Jump up to: 14.0 14.1 (PDF) Advancing Health Through Innovation: New Drug Therapy Approvals 2021 (Report). 13 May 2022. https://www.fda.gov/media/155227/download. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "FDA Approves Syfovre (pegcetacoplan injection) as the First and Only Treatment for Geographic Atrophy (GA), a Leading Cause of Blindness" (Press release). Apellis Pharmaceuticals. 17 February 2023. Archived from the original on 17 February 2023. Retrieved 18 February 2023 – via GlobeNewswire.
- ↑ Jump up to: 16.0 16.1 "Pegcetacoplan: First Approval". Drugs 81 (12): 1423–1430. August 2021. doi:10.1007/s40265-021-01560-8. PMID 34342834.
- ↑ "Comparative effectiveness of pegcetacoplan versus ravulizumab in patients with paroxysmal nocturnal hemoglobinuria previously treated with eculizumab: a matching-adjusted indirect comparison". Current Medical Research and Opinion 37 (11): 1913–1923. November 2021. doi:10.1080/03007995.2021.1971182. PMID 34445916.
- ↑ "Inhibition of C3 with pegcetacoplan results in normalization of hemolysis markers in paroxysmal nocturnal hemoglobinuria". Annals of Hematology 101 (9): 1971–1986. September 2022. doi:10.1007/s00277-022-04903-x. PMID 35869170.
- ↑ "C3 inhibition with pegcetacoplan in subjects with paroxysmal nocturnal hemoglobinuria treated with eculizumab". American Journal of Hematology 95 (11): 1334–1343. November 2020. doi:10.1002/ajh.25960. PMID 33464651.
- ↑ "Pegcetacoplan versus Eculizumab in Paroxysmal Nocturnal Hemoglobinuria". The New England Journal of Medicine 384 (11): 1028–1037. March 2021. doi:10.1056/NEJMoa2029073. PMID 33730455.
- ↑ Jump up to: 21.0 21.1 "Aspaveli: Pending EC decision". European Medicines Agency (EMA). 14 October 2021. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/aspaveli. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Aspaveli Product information". https://ec.europa.eu/health/documents/community-register/html/h1595.htm.
![]() This article incorporates public domain material from the United States Department of Health and Human Services website http://www.fda.gov/.
This article incorporates public domain material from the United States Department of Health and Human Services website http://www.fda.gov/.
External links
- Clinical trial number NCT03500549 for "Study to Evaluate the Efficacy and Safety of APL-2 in Patients With Paroxysmal Nocturnal Hemoglobinuria (PNH)" at ClinicalTrials.gov
- Clinical trial number NCT03525613 for "A Study to Compare the Efficacy and Safety of Intravitreal APL-2 Therapy With Sham Injections in Patients With Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration " at ClinicalTrials.gov
- Clinical trial number NCT03525600 for "Study to Compare the Efficacy and Safety of Intravitreal APL-2 Therapy With Sham Injections in Patients With Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration " at ClinicalTrials.gov
 |

