Chemistry:Potassium telluride
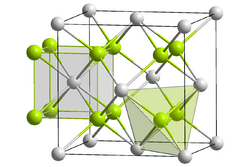 K+: __ Te2-: __
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| K2Te | |
| Molar mass | 298.64 g/mol |
| Melting point | 874 °C |
| Related compounds | |
Other anions
|
Potassium oxide Potassium sulfide Potassium selenide Potassium polonide |
Other cations
|
Lithium telluride Sodium telluride Rubidium telluride Caesium telluride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium telluride is an inorganic compound with a chemical formula K2Te. It is formed from potassium and tellurium, making it a telluride.[1] Potassium telluride is a white powder. Like rubidium telluride and caesium telluride, it can be used as an ultraviolet detector in space. Its crystal structure is similar to other tellurides, which have an anti-fluorite structure.
Production
Tellurium will react with melting potassium cyanide (KCN) producing potassium telluride. It can also be produced by direct combination of potassium and tellurium, usually in liquid ammonia solvent:[2]
- [math]\displaystyle{ \mathrm{2 \ K + Te \longrightarrow K_2Te} }[/math]
Reactions
Adding potassium telluride to water and letting the filtrate stand in air leads to an oxidation reaction that generates potassium hydroxide (KOH) and elemental tellurium:[2][3]
- [math]\displaystyle{ \mathrm{2 \ K_2Te + 2 \ H_2O + O_2 \longrightarrow 4 \ KOH + 2 \ Te} }[/math]
References
- Sangester J. and Pelton AD; Journal of Phase Equilibria, 1997, 18(4) p. 394.
- ↑ Brigitte Eisenmann, Herbert Schäfer: K2Te3 : The First Binary Alkali-Metal Polytelluride with Te2−3-Ions. In: Angewandte Chemie International Edition in English. 17, 1978, S. 684, doi:10.1002/anie.197806841.
- ↑ 2.0 2.1 Wolfgang A. Herrmann, Christian Erich Zybill (2014). Synthetic Methods of Organometallic and Inorganic Chemistry, Volume 4, 1997 Volume 4: Sulfur, Selenium and Tellurium. Georg Thieme Verlag. p. 191. ISBN 978-3-13-179191-7. https://books.google.com/books?id=VIuZAwAAQBAJ.
- ↑ Adolf Pinner (1885) (in German), Repetitorium Der Anorganischen Chemie, Рипол Классик, p. 116, ISBN 978-5-87746-719-4, https://books.google.com/books?id=P8QOAwAAQBAJ&pg=PA116
 |

