Chemistry:Streptomyces isolates
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants.[1] Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.[2]
Anticancer medicines
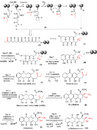
Streptomyces, yielded the medicines doxorubicin (Doxil), daunorubicin (DaunoXome), and streptozotocin (Zanosar). Doxorubicin is the precursor to valrubicin (Valstar), myocet, and pirarubicin. Daunorubicin is the precursor to idarubicin (Idamycin), epirubicin (Ellence), and zorubicin.[citation needed]
Streptomyces is the original source of dactinomycin (Cosmegen), bleomycin (Blenoxane), pingyangmycin (Bleomycin A5), mitomycin C (Mutamycin), rebeccamycin, staurosporine (precursor to stauprimide and midostaurin), neothramycin, aclarubicin, tomaymycin, sibiromycin, and mazethramycin.[citation needed]
Derivatives of Streptomycetes isolate migrastatin, including isomigrastatin, dorrigocin A & B, and the synthetic derivative macroketone, are being researched for anticancer activity.[citation needed]
Antibiotics
Most clinical antibiotics were found during the "golden age of antibiotics" (1940s–1960s). Actinomycin was the first antibiotic isolated from Streptomyces in 1940, followed by streptomycin three years later. Antibiotics from Streptomyces isolates (including various aminoglycosides) would go on to comprise over two-thirds of all marketed antibiotics.[citation needed]
Streptomyces-derived antibiotics include:
- Chloramphenicol (Streptomyces venezuelae)[3]
- Daptomycin (Streptomyces roseosporus)[4]
- Fosfomycin (Streptomyces fradiae)[5]
- Lincomycin (Streptomyces lincolnensis)[6]
- Neomycin (Streptomyces fradiae)[7]
- Platensimycin (Streptomyces platensis)
- Puromycin (Streptomyces alboniger)[8]
- Spenolimycin (Streptomyces gilvospiralis)[9]
- Streptomycin (Streptomyces griseus)[10]
- Tetracycline (Streptomyces rimosus and Streptomyces aureofaciens)[11]
- Kettapeptin[12]
- Niphimycin[13]
Clavulanic acid (Streptomyces clavuligerus) is used in combination with some antibiotics (such as amoxicillin) to weaken bacterial-resistance. Novel anti-infectives being developed include the guadinomines (from Streptomyces sp. K01-0509),[14] inhibitors of the type III secretion system.
Non-Streptomyces actinomycetes, filamentous fungi, and non-filamentous bacteria, have also yielded important antibiotics.[citation needed]
Antifungals
Nystatin (Streptomyces noursei), amphotericin B (Streptomyces nodosus), ossamycin (Streptomyces hygroscopicus), and natamycin (Streptomyces natalensis) are antifungals isolated from Streptomyces.[citation needed]
Immunosuppressants
Sirolimus (Rapamycin), ascomycin, and tacrolimus were isolated from Streptomyces. Pimecrolimus is a derivative of ascomycin. Ubenimex is derived from S. olivoreticuli.[15]
Antiparasitics
Streptomyces avermitilis synthesizes the antiparasitic ivermectin (Stromectol). Other antiparasitics made by Streptomyces include, milbemycin oxime, moxidectin, and milbemycin.[citation needed]
Biotechnology

Traditionally, Escherichia coli is the choice bacterium to express eukaryotic and recombinant genes. E. coli is well understood and has a successful track record producing insulin, the artemisinin precursor artemisinic acid, and filgrastim (Neupogen).[16][17] However, use of E. coli has limitations including misfolding of eukaryotic proteins, insolubility issues, deposition in inclusion bodies, [18] low secretion efficiency, secretion to periplasmic space.
Streptomyces offers potential advantages including superior secretion mechanisms, higher yields, a simpler end-product purification process, making Streptomyces an attractive alternative to E. coli and Bacillus subtilis.[18]
Streptomyces coelicolor, Streptomyces avermitilis, Streptomyces griseus, and Saccharopolyspora erythraea, are capable of secondary metabolite production. Streptomyces coelicolor has shown useful for the heterologous expression of proteins. Methods like "ribosome engineering" have been used to achieve 180-fold higher yields with S. coelicolor.[19]
Other
StreptomeDB, a directory of Streptomyces isolates, contains over 2400 compounds isolated from more than 1900 strains.[20][21] Streptomyces hygroscopicus and Streptomyces viridochromeogenes produce the herbicide bialaphos. Expansion of Streptomyces screenings have included endophytes, extremophiles, and marine varieties.[citation needed]
A recent screening of TCM extracts revealed a Streptomyces that produces a number of antitubercular pluramycins.[22] Wailupemycins are bio-active pyrones isolated from marine Streptomyces.[23]
Mayamycin has been shown to have cytotoxic properties.[24][25]
Germicidin are a group of four compounds that act as autoregulatory inhibitors of spore germination.[26][27]
See also
- Bacillus isolates
- Biotechnology in pharmaceutical manufacturing
- Corynebacterium glutamicum, the industrial/pharmaceutical bacterium responsible for manufacturing a number of amino acids
- Erwinia chrysanthemi, a bacterium used to produce the chemotherapy medicine asparaginase
- Fungal isolates
- Medicinal molds
- Polyene antimycotics
- Sponge isolates
References
- ↑ "How many antibiotics are produced by the genus Streptomyces?". Arch. Microbiol. 176 (5): 386–90. November 2001. doi:10.1007/s002030100345. PMID 11702082. Bibcode: 2001ArMic.176..386W.
- ↑ Schrey, Silvia D. (2012). "Production of fungal and bacterial growth modulating secondary metabolites is widespread among mycorrhiza-associated streptomycetes". BMC Microbiology 12 (1): 164. doi:10.1186/1471-2180-12-164. PMID 22852578.
- ↑ Akagawa, H.; Okanishi, M.; Umezawa, H. (1975). "A Plasmid Involved in Chloramphenicol Production in Streptomyces venezuelae: Evidence from Genetic Mapping". Journal of General Microbiology 90 (2): 336–46. doi:10.1099/00221287-90-2-336. PMID 1194895.
- ↑ Miao, V. (2005). "Daptomycin biosynthesis in Streptomyces roseosporus: Cloning and analysis of the gene cluster and revision of peptide stereochemistry". Microbiology 151 (5): 1507–23. doi:10.1099/mic.0.27757-0. PMID 15870461.
- ↑ "Heterologous production of fosfomycin and identification of the minimal biosynthetic gene cluster". Chemistry & Biology 13 (11): 1171–82. November 2006. doi:10.1016/j.chembiol.2006.09.007. PMID 17113999.
- ↑ Peschke, Ursula; Schmidt, Heike; Zhang, Hui-Zhan; Piepersberg, Wolfgang (1995). "Molecular characterization of the lincomycin-production gene cluster of Streptomyces lincolnensis 78-11". Molecular Microbiology 16 (6): 1137–56. doi:10.1111/j.1365-2958.1995.tb02338.x. PMID 8577249.
- ↑ Howard T. Dulmage (March 1953). "The Production of Neomycin by Streptomyces fradiae in Synthetic Media". Applied Microbiology 1 (2): 103–106. doi:10.1128/AEM.1.2.103-106.1953. PMID 13031516.
- ↑ L. Sankaran; Burton M. Pogell (1975-12-01). "Biosynthesis of Puromycin in Streptomyces alboniger: Regulation and Properties of O-Demethylpuromycin O-Methyltransferase". Antimicrobial Agents and Chemotherapy 8 (6): 721–32. doi:10.1128/AAC.8.6.721. PMID 1211926.
- ↑ Fernandes, Prabhavathi B.; Vojtko, Charlene M.; Bower, Robert R.; Weisz, Jonina (1984). "Spenolimycin, a new spectinomycin-type antibiotic. III. Biological properties.". The Journal of Antibiotics 37 (12): 1525–1527. doi:10.7164/antibiotics.37.1525. PMID 6241193.
- ↑ Distler, Jürgen; Ebert, Andrea; Mansouri, Kambiz; Pissowotzki, Klaus; Stockmann, Michael; Piepersberg, Wolfgang (1987). "Gene cluster for streptomycin biosynthesis inStreptomyces griseus: Nucleotide sequence of three genes and analysis of transcriptional activity". Nucleic Acids Research 15 (19): 8041–56. doi:10.1093/nar/15.19.8041. PMID 3118332.
- ↑ Dr. Mark Nelson; Robert A. Greenwald; Wolfgang Hillen; Mark L. Nelson (2001). Tetracyclines in biology, chemistry and medicine. Birkhäuser. pp. 8–. ISBN 978-3-7643-6282-9. https://books.google.com/books?id=kHNW4tFhZD4C&pg=PA8. Retrieved 17 January 2012.
- ↑ Maskey, RP; Fotso, S; Sevvana, M; Usón, I; Grün-Wollny, I; Laatsch, H (2006). "Kettapeptin: Isolation, structure elucidation and activity of a new hexadepsipeptide antibiotic from a terrestrial Streptomyces sp". The Journal of Antibiotics 59 (5): 309–14. doi:10.1038/ja.2006.44. PMID 16883782.
- ↑ Kim, HY; Kim, JD; Hong, JS; Ham, JH; Kim, BS (2013). "Identification of antifungal niphimycin from Streptomyces sp. KP6107 by screening based on adenylate kinase assay". Journal of Basic Microbiology 53 (7): 581–9. doi:10.1002/jobm.201200045. PMID 22915202.
- ↑ Holmes, TC; May, AE; Zaleta-Rivera, K; Ruby, JG; Skewes-Cox, P; Fischbach, MA; Derisi, JL; Iwatsuki, M et al. (2012). "Molecular insights into the biosynthesis of guadinomine: A type III secretion system inhibitor". Journal of the American Chemical Society 134 (42): 17797–806. doi:10.1021/ja308622d. PMID 23030602.
- ↑ Bauvois, B; Dauzonne, D (January 2006). "Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: Chemistry, biological evaluations, and therapeutic prospects". Medicinal Research Reviews 26 (1): 88–130. doi:10.1002/med.20044. PMID 16216010.
- ↑ "Streptomyces: a host for heterologous gene expression". Curr Opin Biotechnol 2 (5): 674–81. 1991. doi:10.1016/0958-1669(91)90033-2. PMID 1367716.
- ↑ "Improved production of heterologous protein from Streptomyces lividans". Appl Microbiol Biotechnol 33 (4): 395–400. 1990. doi:10.1007/BF00176653. PMID 1369282.
- ↑ 18.0 18.1 "Heterologous biopharmaceutical protein expression in Streptomyces". Trends Biotechnol 15 (8): 315–20. 1997. doi:10.1016/S0167-7799(97)01062-7. PMID 9263479.
- ↑ "Dramatic activation of antibiotic production in Streptomyces coelicolor by cumulative drug resistance mutations.". Appl Environ Microbiol 74 (9): 2834–40. 2008. doi:10.1128/AEM.02800-07. PMID 18310410. Bibcode: 2008ApEnM..74.2834W.
- ↑ "StreptomeDB: a resource for natural compounds isolated from Streptomyces species.". Nucleic Acids Res 41 (Database issue): D1130–6. 2013. doi:10.1093/nar/gks1253. PMID 23193280.
- ↑ "| Pharmaceutical Bioinformatics". 2 November 2018. http://www.pharmaceutical-bioinformatics.de/streptomedb/compound_list/.
- ↑ "Endophytic Streptomyces sp. Y3111 from traditional Chinese medicine produced antitubercular pluramycins.". Appl Microbiol Biotechnol 98 (3): 1077–85. 2014. doi:10.1007/s00253-013-5335-6. PMID 24190497.
- ↑ Kalaitzis, John A (2013). "Discovery, Biosynthesis, and Rational Engineering of Novel Enterocin and Wailupemycin Polyketide Analogues". Metabolomics Tools for Natural Product Discovery. Methods in Molecular Biology. 1055. pp. 171–189. doi:10.1007/978-1-62703-577-4_13. ISBN 978-1-62703-576-7.
- ↑ Bo, Sheng Tao; Xu, Zi Fei; Yang, Li; Cheng, Ping; Tan, Ren Xiang; Jiao, Rui Hua; Ge, Hui Ming (June 2018). "Structure and biosynthesis of mayamycin B, a new polyketide with antibacterial activity from Streptomyces sp. 120454" (in en). The Journal of Antibiotics 71 (6): 601–605. doi:10.1038/s41429-018-0039-x. ISSN 1881-1469. PMID 29515228. https://www.nature.com/articles/s41429-018-0039-x.
- ↑ Schneemann, I; Kajahn, I; Ohlendorf, B; Zinecker, H; Erhard, A; Nagel, K; Wiese, J; Imhoff, J. F. (2010). "Mayamycin, a cytotoxic polyketide from a Streptomyces strain isolated from the marine sponge Halichondria panicea". Journal of Natural Products 73 (7): 1309–12. doi:10.1021/np100135b. PMID 20545334.
- ↑ Aoki, Y; Matsumoto, D; Kawaide, H; Natsume, M (September 2011). "Physiological role of germicidins in spore germination and hyphal elongation in Streptomyces coelicolor A3(2).". The Journal of Antibiotics 64 (9): 607–11. doi:10.1038/ja.2011.59. PMID 21792209.
- ↑ Petersen, F; Zähner, H; Metzger, JW; Freund, S; Hummel, RP (July 1993). "Germicidin, an autoregulative germination inhibitor of Streptomyces viridochromogenes NRRL B-1551". The Journal of Antibiotics 46 (7): 1126–38. doi:10.7164/antibiotics.46.1126. PMID 8360109.
External links
- StreptomeDB
- Antibiotics and Streptomyces : the future of antibiotic discovery
- Bioprospecting for Microbial Endophytes and Their Natural Products - 2003
 |