Chemistry:Ubenimex
| |
| |
| Names | |
|---|---|
| IUPAC name
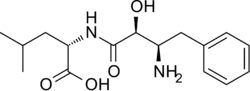
N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutanoyl]-L-leucine
| |
| Systematic IUPAC name
(2S)-2-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutanamido]-4-methylpentanoic acid | |
| Other names
Bestatin; N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl]-L-leucine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H24N2O4 | |
| Molar mass | 308.378 g·mol−1 |
| Melting point | 245 °C (473 °F; 518 K) (decomposes) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B),[3] leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities),[4] alanyl aminopeptidase (aminopeptidase M/N),[5] leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase),[6][7] and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia[8] and lymphedema.[9] It is derived from Streptomyces olivoreticuli.[10] Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.[citation needed]
See also
References
- ↑ N-((2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl)-L-leucine at Sigma-Aldrich
- ↑ "Ubenimex" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/72172#section=Safety-and-Hazards.
- ↑ Umezawa, H.; Aoyagi, T.; Suda, H.; Hamada, M.; Takeuchi, T. (1976). "Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes.". The Journal of Antibiotics 29 (29): 97–99. doi:10.7164/antibiotics.29.97. PMID 931798.
- ↑ Muskardin, D.T.; Voelkel, N.F.; Fitzpatrick, F.A. (1994). "Modulation of pulmonary leukotriene formation and perfusion pressure by Bestatin, an inhibitor of leukotriene A4 hydrolase.". Biochemical Pharmacology 48 (48): 131–137. doi:10.1016/0006-2952(94)90232-1. PMID 8043014.
- ↑ K Sekine; H Fujii; F Abe (1999). "Induction of apoptosis by Bestatin (ubenimex) in human leukemic cell lines". Leukemia 13 (5): 729–734. doi:10.1038/sj.leu.2401388. PMID 10374877.
- ↑ "Immunoaffinity purification and characterization of native placental leucine aminopeptidase/oxytocinase from human placenta". Placenta 21 (7): 628–34. 2000. doi:10.1053/plac.2000.0564. PMID 10985965.
- ↑ "Oxytocin is hydrolyzed by an enzyme in human placenta that is identical to the oxytocinase of pregnancy serum". Peptides 17 (2): 257–61. 1996. doi:10.1016/0196-9781(95)02124-8. PMID 8801531.
- ↑ Hirayama, Y; Sakamaki, S; Takayanagi, N; Tsuji, Y; Sagawa, T; Chiba, H; Matsunaga, T; Niitsu, Y (2003). "Chemotherapy with ubenimex corresponding to patient age and organ disorder for 18 cases of acute myelogeneous leukemia in elderly patients--effects, complications and long-term survival". Gan to Kagaku Ryoho. Cancer & Chemotherapy 30 (8): 1113–8. PMID 12938265.
- ↑ Tian, W; Rockson, S; Jiang, X; Kim, J; Begaye, A; Shuffle, EM; Tu, AB; Cribb, M et al. (2017). "Leukotriene B4 antagonism ameliorates experimental lymphedema". Science Translational Medicine 9 (389): eaal3920. doi:10.1126/scitranslmed.aal3920. PMID 28490670.
- ↑ Bauvois, B; Dauzonne, D (January 2006). "Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: Chemistry, biological evaluations, and therapeutic prospects". Medicinal Research Reviews 26 (1): 88–130. doi:10.1002/med.20044. PMID 16216010.
External links
 |
