Philosophy:Neurobiological effects of physical exercise
The neurobiological effects of physical exercise involve possible interrelated effects on brain structure, brain function, and cognition.[1][2][3][4] Research in humans has demonstrated that consistent aerobic exercise (e.g., 30 minutes every day) may induce improvements in certain cognitive functions, neuroplasticity and behavioral plasticity; some of these long-term effects may include increased neuron growth, increased neurological activity (e.g., c-Fos and BDNF signaling), improved stress coping, enhanced cognitive control of behavior, improved declarative, spatial, and working memory, and structural and functional improvements in brain structures and pathways associated with cognitive control and memory.[5][6][7] The effects of exercise on cognition may affect academic performance in children and college students, improve adult productivity, preserve cognitive function in old age, preventing or treating certain neurological disorders, and improving overall quality of life.[8][9][10][11]
In healthy adults, aerobic exercise has been shown to induce transient effects on cognition after a single exercise session and persistent effects on cognition following consistent exercise over the course of several months.[1][7][12] People who regularly perform an aerobic exercise (e.g., running, jogging, brisk walking, swimming, and cycling) have greater scores on neuropsychological function and performance tests that measure certain cognitive functions, such as attentional control, inhibitory control, cognitive flexibility, working memory updating and capacity, declarative memory, spatial memory, and information processing speed.[5][7][12][13][14]
Aerobic exercise has both short and long term effects on mood and emotional states by promoting positive affect, inhibiting negative affect, and decreasing the biological response to acute psychological stress.[12] Aerobic exercise may affect both self-esteem and overall well-being (including sleep patterns) with consistent, long term participation.[15] Regular aerobic exercise may improve symptoms associated with central nervous system disorders and may be used as adjunct therapy for these disorders. There is some evidence of exercise treatment efficacy for major depressive disorder and attention deficit hyperactivity disorder.[9][16][17][18] The American Academy of Neurology's clinical practice guideline for mild cognitive impairment indicates that clinicians should recommend regular exercise (two times per week) to individuals who have been diagnosed with this condition.[19]
Some preclinical evidence and emerging clinical evidence supports the use of exercise as an adjunct therapy for the treatment and prevention of drug addictions.[20][21][22][23]
Reviews of clinical evidence also support the use of exercise as an adjunct therapy for certain neurodegenerative disorders, particularly Alzheimer's disease and Parkinson's disease.[24][25] Regular exercise may be associated with a lower risk of developing neurodegenerative disorders.[26]
Long-term effects
Neuroplasticity
Neuroplasticity is the process by which neurons adapt to a disturbance over time, and most often occurs in response to repeated exposure to stimuli.[27] Aerobic exercise increases the production of neurotrophic factors[note 1] (e.g., BDNF, IGF-1, VEGF) which mediate improvements in cognitive functions and various forms of memory by promoting blood vessel formation in the brain, adult neurogenesis,[note 2] and other forms of neuroplasticity.[2][5][29][30] Consistent aerobic exercise over a period of several months induces clinically significant improvements in executive functions and increased gray matter volume in nearly all regions of the brain,[31] with the most marked increases occurring in brain regions that give rise to executive functions.[1][5][6] The brain structures that show the greatest improvements in gray matter volume in response to aerobic exercise are the prefrontal cortex, caudate nucleus, and hippocampus;[1][5] less significant increases in gray matter volume occur in the anterior cingulate cortex, parietal cortex, cerebellum, and nucleus accumbens.[5] The prefrontal cortex, caudate nucleus, and anterior cingulate cortex are among the most significant brain structures in the dopamine and norepinephrine systems that give rise to cognitive control.[32] Exercise-induced neurogenesis (i.e., the increases in gray matter volume) in the hippocampus is associated with measurable improvements in spatial memory.[33][34] Higher physical fitness scores, as measured by VO2 max, are associated with better executive function, faster information processing speed, and greater gray matter volume of the hippocampus, caudate nucleus, and nucleus accumbens.[1]
Structural growth
Reviews of neuroimaging studies indicate that consistent aerobic exercise increases gray matter volume in nearly all regions of the brain,[31] with more pronounced increases occurring in brain regions associated with memory processing, cognitive control, motor function, and reward;[1][5][31] the most prominent gains in gray matter volume are seen in the prefrontal cortex, caudate nucleus, and hippocampus, which support cognitive control and memory processing, among other cognitive functions.[1][6] Moreover, the left and right halves of the prefrontal cortex, the hippocampus, and the cingulate cortex appear to become more functionally interconnected in response to consistent aerobic exercise.[1] Three reviews indicate that marked improvements in prefrontal and hippocampal gray matter volume occur in healthy adults that regularly engage in medium intensity exercise for several months.[1][35] Other regions of the brain that demonstrate moderate or less significant gains in gray matter volume during neuroimaging include the anterior cingulate cortex, parietal cortex, cerebellum, and nucleus accumbens.[5][36]
Regular exercise has been shown to counter the shrinking of the hippocampus and memory impairment that naturally occurs in late adulthood.[5] Sedentary adults over age 55 show a 1–2% decline in hippocampal volume annually.[37] A neuroimaging study with a sample of 120 adults revealed that participating in regular aerobic exercise increased the volume of the left hippocampus by 2.12% and the right hippocampus by 1.97% over a one-year period.[37] Subjects in the low intensity stretching group who had higher fitness levels at baseline showed less hippocampal volume loss, providing evidence for exercise being protective against age-related cognitive decline.[37] In general, individuals that exercise more over a given period have greater hippocampal volumes and better memory function.[5] Aerobic exercise has also been shown to induce growth in the white matter tracts in the anterior corpus callosum, which normally shrink with age.[5][35]
The various functions of the brain structures that show exercise-induced increases in gray matter volume include:
- Caudate nucleus – responsible for stimulus-response learning and inhibitory control; implicated in Parkinson's disease and ADHD[38][39]
- Cerebellum – responsible for motor coordination and motor learning[40]
- Hippocampus – responsible for storage and consolidation of declarative memory and spatial memory[39]
- Nucleus accumbens – responsible for incentive salience ("wanting" or desire, the form of motivation associated with reward) and positive reinforcement; implicated in addiction[41]
- Parietal cortex – responsible for sensory perception, working memory, and attention[38][42]
- Prefrontal and anterior cingulate cortices – required for the cognitive control of behavior, particularly: working memory, attentional control, decision-making, cognitive flexibility, social cognition, and inhibitory control of behavior;[38][43] implicated in attention deficit hyperactivity disorder (ADHD) and addiction[38]
Persistent effects on cognition
Concordant with the functional roles of the brain structures that exhibit increased gray matter volumes, regular exercise over a period of several months has been shown to persistently improve numerous executive functions and several forms of memory.[5][6][44][45] In particular, consistent aerobic exercise has been shown to improve attentional control,[note 3] information processing speed, cognitive flexibility (e.g., task switching), inhibitory control,[note 4] working memory updating and capacity,[note 5] declarative memory,[note 6] and spatial memory.[5][6][7][44] In healthy young and middle-aged adults, the effect sizes of improvements in cognitive function are largest for indices of executive functions and small to moderate for aspects of memory and information processing speed.[1][7] It may be that in older adults, individuals benefit cognitively by taking part in both aerobic and resistance type exercise of at least moderate intensity.[47] Individuals who have a sedentary lifestyle tend to have impaired executive functions relative to other more physically active non-exercisers.[6] A reciprocal relationship between exercise and executive functions has also been noted: improvements in executive control processes, such as attentional control and inhibitory control, increase an individual's tendency to exercise.[6]
Mechanism of effects
BDNF signaling
One of the most significant effects of exercise on the brain is increased synthesis and expression of BDNF, a neuropeptide and hormone, resulting in increased signaling through its receptor tyrosine kinase, tropomyosin receptor kinase B (TrkB).[4][48][49] Since BDNF is capable of crossing the blood–brain barrier, higher peripheral BDNF synthesis also increases BDNF signaling in the brain.[30] Exercise-induced increases in BDNF signaling are associated with improved cognitive function, improved mood, and improved memory.[29][48] Furthermore, research has provided a great deal of support for the role of BDNF in hippocampal neurogenesis, synaptic plasticity, and neural repair.[5][48] Engaging in moderate-high intensity aerobic exercise such as running, swimming, and cycling increases BDNF biosynthesis through myokine signaling, resulting in up to a threefold increase in blood plasma and BDNF levels;[4][48][49] exercise intensity is positively correlated with the magnitude of increased BDNF biosynthesis and expression.[4][48][49] A meta-analysis of studies involving the effect of exercise on BDNF levels found that consistent exercise modestly increases resting BDNF levels as well.[29] This has important implications for exercise as a mechanism to reduce stress since stress is closely linked with decreased levels of BDNF in the hippocampus. In fact, studies suggest that BDNF contributes to the anxiety-reducing effects of antidepressants. The increase in BDNF levels caused by exercise helps reverse the stress-induced decrease in BDNF which mediates stress in the short term and buffers against stress-related diseases in the long term.[50]
IGF-1 signaling
IGF-1 is a peptide and neurotrophic factor that mediates some of the effects of growth hormone;[51] IGF-1 elicits its physiological effects by binding to a specific receptor tyrosine kinase, the IGF-1 receptor, to control tissue growth and remodeling.[51] In the brain, IGF-1 functions as a neurotrophic factor that, like BDNF, plays a significant role in cognition, neurogenesis, and neuronal survival.[48][52][53] Physical activity is associated with increased levels of IGF-1 in blood serum, which is known to contribute to neuroplasticity in the brain due to its capacity to cross the blood–brain barrier and blood–cerebrospinal fluid barrier;[5][48][51][52] consequently, one review noted that IGF-1 is a key mediator of exercise-induced adult neurogenesis, while a second review characterized it as a factor which links "body fitness" with "brain fitness".[51][52] The amount of IGF-1 released into blood plasma during exercise is positively correlated with exercise intensity and duration.[54]
VEGF signaling
VEGF is a neurotrophic and angiogenic (i.e., blood vessel growth-promoting) signaling protein that binds to two receptor tyrosine kinases, VEGFR1 and VEGFR2, which are expressed in neurons and glial cells in the brain.[53] Hypoxia, or inadequate cellular oxygen supply, strongly upregulates VEGF expression and VEGF exerts a neuroprotective effect in hypoxic neurons.[53] Like BDNF and IGF-1, aerobic exercise has been shown to increase VEGF biosynthesis in peripheral tissue which subsequently crosses the blood–brain barrier and promotes neurogenesis and blood vessel formation in the central nervous system.[30][55] Exercise-induced increases in VEGF signaling have been shown to improve cerebral blood volume and contribute to exercise-induced neurogenesis in the hippocampus.[5][55]
Irisin
A study using FNDC5 knock-out mice as well as artificial elevation of circulating irisin levels showed that irisin confers beneficial cognitive effects of physical exercise and that it can serve an exercise mimetic in mice in which it could "improve both the cognitive deficit and neuropathology in Alzheimer's disease mouse models". The mediator and its regulatory system is therefore being investigated for potential interventions to improve – or further improve – cognitive function or alleviate Alzheimer's disease in humans.[56][57][58] Experiments indicate irisin may be linked to regulation of BDNF and neurogenesis in mice.[59]
Short-term effects
Transient effects on cognition
In addition to the persistent effects on cognition that result from several months of daily exercise, acute exercise (i.e., a single bout of exercise) has been shown to transiently improve a number of cognitive functions.[12][60][61] Reviews and meta-analyses of research on the effects of acute exercise on cognition in healthy young and middle-aged adults have concluded that information processing speed and a number of executive functions – including attention, working memory, problem solving, cognitive flexibility, verbal fluency, decision making, and inhibitory control – all improve for a period of up to 2 hours post-exercise.[12][60][61] A systematic review of studies conducted on children also suggested that some of the exercise-induced improvements in executive function are apparent after single bouts of exercise, while other aspects (e.g., attentional control) only improve following consistent exercise on a regular basis.[44] Other research has suggested immediate performative enhancements during exercise, such as exercise-concurrent improvements in processing speed and accuracy during both visual attention and working memory tasks.[62][63]
Exercise-induced euphoria
It has been suggested that this section be split out into another page titled Runner's high. (Discuss) (April 2021) |
Continuous exercise can produce a transient state of euphoria – a positively-valenced affective state involving the experience of pleasure and feelings of profound contentment, elation, and well-being – which is colloquially known as a "runner's high" in distance running or a "rower's high" in rowing.[64][65][66][67] Current medical reviews indicate that several endogenous euphoriants are responsible for producing exercise-related euphoria, specifically phenethylamine (an endogenous psychostimulant), β-endorphin (an endogenous opioid), and anandamide (an endogenous cannabinoid).[68][69][70][71][72]
Effects on neurochemistry
β-Phenylethylamine
{{Annotated image 4 | caption = {{{caption|In humans, catecholamines and phenethylaminergic trace amines are derived from the amino acid {{nowrap|L-phenylalanine}}.}}} | header_background = #F0F8FF | header = Biosynthetic pathways for catecholamines and trace amines in the human brain<ref name="Trace amine template 1">Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacol. Ther. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.</ref>[73][74] | alt = Graphic of catecholamine and trace amine biosynthesis | image = Catecholamine and trace amine biosynthesis.png | image-width = 580 | image-left = 5 | image-top = 0 | align = right | width = 590 | height = 585 | annot-font-size = 14 | annot-text-align = center | annotations =
{{annotation|50|565|{{if pagename|Adrenaline=Adrenaline|Epinephrine=Epinephrine|Catecholamine=Epinephrine|other=Epinephrine}}}}
{{annotation|245|60|{{if pagename|Phenethylamine=Phenethylamine|Trace amine=Phenethylamine|Neurobiological effects of physical exercise={{highlight|Phenethylamine}}|other=Phenethylamine}}}}
{{annotation|245|565|{{if pagename|Norepinephrine=Norepinephrine|Adrenaline=Noradrenaline|Catecholamine=Norepinephrine|other=Norepinephrine}}}}
{{annotation|440|295|p-Octopamine}}}}
pathway
CYP2D6
pathway
β-Phenylethylamine, commonly referred to as phenethylamine, is a human trace amine and potent catecholaminergic and glutamatergic neuromodulator that has similar psychostimulant and euphoriant effects and a similar chemical structure to amphetamine.[75] Thirty minutes of moderate to high intensity physical exercise has been shown to induce an enormous increase in urinary β-phenylacetic acid, the primary metabolite of phenethylamine.[68][69][70] Two reviews noted a study where the average 24 hour urinary β-phenylacetic acid concentration among participants following just 30 minutes of intense exercise increased by 77% relative to baseline concentrations in resting control subjects;[68][69][70] the reviews suggest that phenethylamine synthesis sharply increases while an individual is exercising, during which time it is rapidly metabolized due to its short half-life of roughly 30 seconds.[68][69][70][76] In a resting state, phenethylamine is synthesized in catecholamine neurons from L-phenylalanine by aromatic amino acid decarboxylase (AADC) at approximately the same rate at which dopamine is produced.[76]
In light of this observation, the original paper and both reviews suggest that phenethylamine plays a prominent role in mediating the mood-enhancing euphoric effects of a runner's high, as both phenethylamine and amphetamine are potent euphoriants.[68][69][70]
β-Endorphin
β-Endorphin (contracted from "endogenous morphine") is an endogenous opioid neuropeptide that binds to μ-opioid receptors, in turn producing euphoria and pain relief.[71] A meta-analytic review found that exercise significantly increases the secretion of β-endorphin and that this secretion is correlated with improved mood states.[71] Moderate intensity exercise produces the greatest increase in β-endorphin synthesis, while higher and lower intensity forms of exercise are associated with smaller increases in β-endorphin synthesis.[71] A review on β-endorphin and exercise noted that an individual's mood improves for the remainder of the day following physical exercise and that one's mood is positively correlated with overall daily physical activity level.[71]
However, humans studies showed that pharmacological blockade of endogenous endorphins does not inhibit a runner's high, while blockade of endocannabinoids may have such an effect.[77]
Anandamide
Anandamide is an endogenous cannabinoid and retrograde neurotransmitter that binds to cannabinoid receptors (primarily CB1), in turn producing euphoria.[66][72] It has been shown that aerobic exercise causes an increase in plasma anandamide levels, where the magnitude of this increase is highest at moderate exercise intensity (i.e., exercising at ~70–80% maximum heart rate).[72] Increases in plasma anandamide levels are associated with psychoactive effects because anandamide is able to cross the blood–brain barrier and act within the central nervous system.[72] Thus, because anandamide is a euphoriant and aerobic exercise is associated with euphoric effects, it has been proposed that anandamide partly mediates the short-term mood-lifting effects of exercise (e.g., the euphoria of a runner's high) via exercise-induced increases in its synthesis.[66][72]
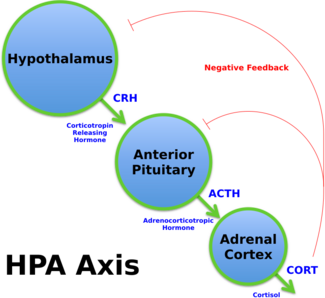
Cortisol and the psychological stress response
The "stress hormone", cortisol, is a glucocorticoid that binds to glucocorticoid receptors.[78][79][80] Psychological stress induces the release of cortisol from the adrenal gland by activating the hypothalamic–pituitary–adrenal axis (HPA axis).[78][79][80] Short-term increases in cortisol levels are associated with adaptive cognitive improvements, such as enhanced inhibitory control;[79][80] however, excessively high exposure or prolonged exposure to high levels of cortisol causes impairments in cognitive control and has neurotoxic effects in the human brain.[80] For example, chronic psychological stress decreases BDNF expression, which has detrimental effects on hippocampal volume and can lead to depression.[78]
As a physical stressor, aerobic exercise stimulates cortisol secretion in an intensity-dependent manner;[79] however, it does not result in long-term increases in cortisol production since this exercise-induced effect on cortisol is a response to transient negative energy balance.[note 7][79] Aerobic exercise increases physical fitness and lowers neuroendocrine (i.e., HPA axis) reactivity and therefore reduces the biological response to psychological stress in humans (e.g., reduced cortisol release and attenuated heart rate response).[12][81] Exercise also reverses stress-induced decreases in BDNF expression and signaling in the brain, thereby acting as a buffer against stress-related diseases like depression.[78][81]
Glutamate and GABA
Glutamate, one of the most common neurochemicals in the brain, is an excitatory neurotransmitter involved in many aspects of brain function, including learning and memory.[82] Based upon animal models, exercise appears to normalize the excessive levels of glutamate neurotransmission into the nucleus accumbens that occurs in drug addiction.[21] A review of the effects of exercise on neurocardiac function in preclinical models noted that exercise-induced neuroplasticity of the rostral ventrolateral medulla (RVLM) has an inhibitory effect on glutamatergic neurotransmission in this region, in turn reducing sympathetic activity;[83] the review hypothesized that this neuroplasticity in the RVLM is a mechanism by which regular exercise prevents inactivity-related cardiovascular disease.[83]
Exerkines and other circulating compounds
Exerkines are putative "signalling moieties released in response to acute and/or chronic exercise, which exert their effects through endocrine, paracrine and/or autocrine pathways".[84]
Effects in children
Engaging in active physical pursuits has demonstrated positive effects on the mental health of children and adolescents,[85] enhances their academic performance,[86] boosts cognitive function,[87] and diminishes the likelihood of obesity and cardiovascular diseases among this demographic.[88] Establishing consistent exercise routines with regular frequency and duration is pivotal.[89][90][91] Cultivating beneficial exercise habits and sustaining adequate physical activity may support the overall physical and mental well-being of young individuals. Therefore, identifying factors that either impede or encourage exercise behaviors could be a significant strategy in promoting the development of healthy exercise habits among children and adolescents.
A 2003 meta-analysis found a positive effect of exercise in children on perceptual skills, intelligence quotient, achievement, verbal tests, mathematic tests, and academic readiness.[92] The correlation was strongest for the age ranges of 4–7 and 11–13 years.[92]
A 2010 meta-analysis of the effect of activity on children's executive function found that aerobic exercise may briefly aid children's executive function and also influence more lasting improvements to executive function.[93] Other studies suggested that exercise is unrelated to academic performance, perhaps due to the parameters used to determine exactly what academic achievement is.[94] This area of study has been a focus for education boards that make decisions on whether physical education should be implemented in the school curriculum, how much time should be dedicated to physical education, and its impact on other academic subjects.[92]
Another study found that sixth-graders who participated in vigorous physical activity at least three times a week had the highest scores compared to those who participated in moderate or no physical activity at all. Children who participated in vigorous physical activity scored three points higher, on average, on their academic test, which consisted of math, science, English, and world studies.[95]
Neuroimaging studies indicate that exercise may influence changes in brain structure and function.[94] Some investigations have linked low levels of aerobic fitness in children with impaired executive function when older as adults, but lack of selective attention, response inhibition, and interference control may also explain this outcome.[96]
Effects on central nervous system disorders
Exercise as prevention and treatment of drug addictions
Clinical and preclinical evidence indicate that consistent aerobic exercise, especially endurance exercise (e.g., marathon running), actually prevents the development of certain drug addictions and is an effective adjunct treatment for drug addiction, and psychostimulant addiction in particular.[20][21][22][23] Consistent aerobic exercise magnitude-dependently (i.e., by duration and intensity) may reduce drug addiction risk, which appears to occur through the reversal of drug-induced, addiction-related neuroplasticity.[21][22] Moreover, aerobic exercise decreases psychostimulant self-administration, reduces the reinstatement (i.e., relapse) of drug-seeking, and induces opposite effects on striatal dopamine receptor D2 (DRD2) signaling (increased DRD2 density) to those induced by pathological stimulant use (decreased DRD2 density).[21][22] Consequently, consistent aerobic exercise may lead to better treatment outcomes when used as an adjunct treatment for drug addiction.[21][23] (As of 2016), more clinical research is still needed to understand the mechanisms and confirm the efficacy of exercise in drug addiction treatment and prevention.[20]
| Form of neuroplasticity or behavioral plasticity |
Type of reinforcer | Sources | |||||
|---|---|---|---|---|---|---|---|
| Opiates | Psychostimulants | High fat or sugar food | Sexual intercourse | Physical exercise (aerobic) |
Environmental enrichment | ||
| ΔFosB expression in nucleus accumbens D1-type MSNs |
↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [22] |
| Behavioral plasticity | |||||||
| Escalation of intake | Yes | Yes | Yes | [22] | |||
| Psychostimulant cross-sensitization |
Yes | Not applicable | Yes | Yes | Attenuated | Attenuated | [22] |
| Psychostimulant self-administration |
↑ | ↑ | ↓ | ↓ | ↓ | [22] | |
| Psychostimulant conditioned place preference |
↑ | ↑ | ↓ | ↑ | ↓ | ↑ | [22] |
| Reinstatement of drug-seeking behavior | ↑ | ↑ | ↓ | ↓ | [22] | ||
| Neurochemical plasticity | |||||||
| CREB phosphorylation in the nucleus accumbens |
↓ | ↓ | ↓ | ↓ | ↓ | [22] | |
| Sensitized dopamine response in the nucleus accumbens |
No | Yes | No | Yes | [22] | ||
| Altered striatal dopamine signaling | ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD2 | ↑DRD2 | [22] | |
| Altered striatal opioid signaling | No change or ↑μ-opioid receptors |
↑μ-opioid receptors ↑κ-opioid receptors |
↑μ-opioid receptors | ↑μ-opioid receptors | No change | No change | [22] |
| Changes in striatal opioid peptides | ↑dynorphin No change: enkephalin |
↑dynorphin | ↓enkephalin | ↑dynorphin | ↑dynorphin | [22] | |
| Mesocorticolimbic synaptic plasticity | |||||||
| Number of dendrites in the nucleus accumbens | ↓ | ↑ | ↑ | [22] | |||
| Dendritic spine density in the nucleus accumbens |
↓ | ↑ | ↑ | [22] | |||
Attention deficit hyperactivity disorder
Regular physical exercise, particularly aerobic exercise, is an effective add-on treatment for ADHD in children and adults, particularly when combined with stimulant medication (i.e., amphetamine or methylphenidate), although the best intensity and type of aerobic exercise for improving symptoms are not currently known.[18][97] In particular, the long-term effects of regular aerobic exercise in ADHD individuals include better behavior and motor abilities, improved executive functions (including attention, inhibitory control, and planning, among other cognitive domains), faster information processing speed, and better memory.[18] Parent-teacher ratings of behavioral and socio-emotional outcomes in response to regular aerobic exercise include: better overall function, reduced ADHD symptoms, better self-esteem, reduced levels of anxiety and depression, fewer somatic complaints, better academic and classroom behavior, and improved social behavior.[18] Exercising while on stimulant medication augments the effect of stimulant medication on executive function.[18] It is believed that these short-term effects of exercise are mediated by an increased abundance of synaptic dopamine and norepinephrine in the brain.[18]
Major depressive disorder
A number of medical reviews have indicated that exercise has a marked and persistent antidepressant effect in humans,[5][16][98][17][99][100] an effect believed to be mediated through enhanced BDNF signaling in the brain.[17] Several systematic reviews have analyzed the potential for physical exercise in the treatment of depressive disorders. The 2013 Cochrane Collaboration review on physical exercise for depression noted that, based upon limited evidence, it is more effective than a control intervention and comparable to psychological or antidepressant drug therapies.[99] Three subsequent 2014 systematic reviews that included the Cochrane review in their analysis concluded with similar findings: one indicated that physical exercise is effective as an adjunct treatment (i.e., treatments that are used together) with antidepressant medication;[17] the other two indicated that physical exercise has marked antidepressant effects and recommended the inclusion of physical activity as an adjunct treatment for mild–moderate depression and mental illness in general.[16][98] One systematic review noted that yoga may be effective in alleviating symptoms of prenatal depression.[101] Another review asserted that evidence from clinical trials supports the efficacy of physical exercise as a treatment for depression over a 2–4 month period.[5] These benefits have also been noted in old age, with a review conducted in 2019 finding that exercise is an effective treatment for clinically diagnosed depression in older adults.[102]
A meta-analysis from July 2016 concluded that physical exercise improves overall quality of life in individuals with depression relative to controls.[9][103]
Cerebrovascular disease
Physical exercise plays a significant role in the prevention and management of stroke. It is well established that physical activity decrease the risk of ischemic stroke and intracerebral haemorrhage.[104][105] Engaging in physical activity before experiencing a stroke has been found to have a positive impact on the severity and outcomes of stroke.[106] Exercise has the potential to increase the expression of VEGF, caveolin, and angiopoietin in the brain. These changes may promote angiogenesis and neovascularization that contribute to improved blood supply to the stroke affected areas of the brain.[107][108][109] Exercise may affect the activation of endothelial nitric oxide synthase (eNOS) and subsequent production of nitric oxide (NO).[110][111][112] The increase in NO production may lead to improved post-stroke cerebral blood flow, ensuring a sufficient oxygen and nutrient supply to the brain. Physical activity has been associated with increased expression and activation of hypoxia-inducible factor 1 alpha (HIF-1α), heat shock proteins, and brain-derived neurotrophic factor (BDNF).[113][114][115] These factors play crucial roles in promoting cellular survival, neuroprotection, and repair processes in the brain following a stroke. Exercise also inhibit glutamate and caspase activities, which are involved in neuronal death pathways.[116][117][118][119] Additionally, it may promote neurogenesis in the brain. These effects collectively contribute to the reduction of brain infarction and edema, leading to potential improvements in neurological and functional outcomes. The neuroprotective properties of physical activity in relation to haemorrhagic strokes are less studied. Pre-stroke physical activity has been associated with improved outcomes after intracerebral haemorrhages.[120] Furthermore, physical activity may reduce the volume of intracerebral haemorrhages.[121][122] Being physically active after stroke also enhance the functional recovery.[123][124][125]
Mild cognitive impairment
The American Academy of Neurology's January 2018 update of their clinical practice guideline for mild cognitive impairment states that clinicians should recommend regular exercise (two times per week) to individuals who have been diagnosed with this condition.[19] This guidance is based upon a moderate amount of high-quality evidence which supports the efficacy of regular physical exercise (twice weekly over a 6-month period) for improving cognitive symptoms in individuals with mild cognitive impairment.[19]
Neurodegenerative disorders
Alzheimer's disease
Alzheimer's disease is a cortical neurodegenerative disorder and the most prevalent form of dementia, representing approximately 65% of all cases of dementia; it is characterized by impaired cognitive function, behavioral abnormalities, and a reduced capacity to perform basic activities of daily life.[24] Two reviews found evidence for possible positive effects of physical exercise on cognitive function, the rate of cognitive decline, and the ability to perform activities of daily living in individuals with Alzheimer's disease.[24] A subsequent review found higher levels of physical activity may be associated with reduced risk of dementia and cognitive decline.[26]
Parkinson's disease
Parkinson's disease symptoms reflect various functional impairments and limitations, such as postural instability, gait disturbance, immobility, and frequent falls. Some evidence suggests that physical exercise may lower the risk of Parkinson's disease.[126] A 2017 study found that strength and endurance training in people with Parkinson's disease had positive effects lasting for several weeks.[127] A 2023 Cochrane review on the effects of physical exercise in people with Parkinson's disease indicated that aquatic exercise might reduce severity of motor symptoms and improve quality of life.[128] Furthermore, endurance training, functional training, and multi-domain training (i.e., engaging in several types of exercise) may provide improvements.[128]
See also
- Brain fitness
- Exercise is Medicine
- Exercise prescription
- Exercise therapy
- Memory improvement
- Neuroinflammation
- Nootropic
Notes
- ↑ Neurotrophic factors are peptides or other small proteins that promote the growth, survival, and differentiation of neurons by binding to and activating their associated tyrosine kinases.[28]
- ↑ Adult neurogenesis is the postnatal (after-birth) growth of new neurons, a beneficial form of neuroplasticity.[27]
- ↑ Attentional control allows an individual to focus their attention on a specific source and ignore other stimuli that compete for one's attention,[32] such as in the cocktail party effect.
- ↑ Inhibitory control is the process of altering one's learned behavioral responses, sometimes called "prepotent responses", in a way that makes it easier to complete a particular goal.[38][46] Inhibitory control allows individuals to control their impulses and habits when necessary or desired,[38][46] e.g., to overcome procrastination.
- ↑ Working memory is the form of memory used by an individual at any given moment for active information processing,[32] such as when reading or writing an encyclopedia article. Working memory has a limited capacity and functions as an information buffer, analogous to a computer's data buffer, that permits the manipulation of information for comprehension, decision-making, and guidance of behavior.[38]
- ↑ Declarative memory, also known as explicit memory, is the form of memory that pertains to facts and events.[39]
- ↑ In healthy individuals, this energy deficit resolves simply from eating and drinking a sufficient amount of food and beverage after exercising.
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 "Physical activity, brain, and cognition". Current Opinion in Behavioral Sciences 4: 27–32. August 2015. doi:10.1016/j.cobeha.2015.01.005.
- ↑ Jump up to: 2.0 2.1 "Protective Effects of Physical Exercise in Alzheimer's Disease and Parkinson's Disease: A Narrative Review". J Clin Neurol 11 (3): 212–219. July 2015. doi:10.3988/jcn.2015.11.3.212. PMID 26174783.
- ↑ "The neuropathology of sport". Acta Neuropathol. 127 (1): 29–51. January 2014. doi:10.1007/s00401-013-1230-6. PMID 24366527.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 "Exercise: putting action into our epigenome". Sports Med 44 (2): 189–209. February 2014. doi:10.1007/s40279-013-0114-1. PMID 24163284.
- ↑ Jump up to: 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 "The influence of exercise on cognitive abilities". Comprehensive Physiology 3 (1): 403–428. January 2013. doi:10.1002/cphy.c110063. ISBN 9780470650714. PMID 23720292.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 6.5 6.6 "Cognitive control in the self-regulation of physical activity and sedentary behavior". Front Hum Neurosci 8: 747. 2014. doi:10.3389/fnhum.2014.00747. PMID 25324754.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 "Relationship between physical activity and cognitive function in apparently healthy young to middle-aged adults: A systematic review". J. Sci. Med. Sport 19 (8): 616–628. August 2016. doi:10.1016/j.jsams.2015.09.003. PMID 26552574.
- ↑ CDC (2023-08-01). "Benefits of Physical Activity" (in en-us). https://www.cdc.gov/physicalactivity/basics/pa-health/index.htm.
- ↑ Jump up to: 9.0 9.1 9.2 "Exercise improves physical and psychological quality of life in people with depression: A meta-analysis including the evaluation of control group response". Psychiatry Res. 241: 47–54. July 2016. doi:10.1016/j.psychres.2016.04.054. PMID 27155287. https://kclpure.kcl.ac.uk/portal/en/publications/9d2c5d0c-a5a9-4e46-a763-6b1398bce0f6.
- ↑ "Motor Activity in Aging: An Integrated Approach for Better Quality of Life". International Scholarly Research Notices 2014: 257248. November 2014. doi:10.1155/2014/257248. PMID 27351018.
- ↑ "Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits". Frontiers in Psychology 9: 509. 27 April 2018. doi:10.3389/fpsyg.2018.00509. PMID 29755380.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 12.5 "The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review". Brain Plasticity 2 (2): 127–152. March 2017. doi:10.3233/BPL-160040. PMID 29765853.
- ↑ "Exercise and mental health" (in en). Department of Health & Human Services. http://www.betterhealth.vic.gov.au/health/healthyliving/exercise-and-mental-health.
- ↑ "Exercise and Mental Health". Exercise Psychology: 93–94. 2013. doi:10.5040/9781492595502.part-002. ISBN 9781492595502. http://dx.doi.org/10.5040/9781492595502.part-002.
- ↑ "10 great reasons to love aerobic exercise" (in en). https://www.mayoclinic.org/healthy-lifestyle/fitness/in-depth/aerobic-exercise/art-20045541.
- ↑ Jump up to: 16.0 16.1 16.2 "Physical exercise intervention in depressive disorders: meta-analysis and systematic review". Scand J Med Sci Sports 24 (2): 259–272. 2014. doi:10.1111/sms.12050. PMID 23362828.
- ↑ Jump up to: 17.0 17.1 17.2 17.3 "Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review". CNS Spectr 19 (6): 496–508. 2014. doi:10.1017/S1092852913000953. PMID 24589012.
- ↑ Jump up to: 18.0 18.1 18.2 18.3 18.4 18.5 "Sweat it out? The effects of physical exercise on cognition and behavior in children and adults with ADHD: a systematic literature review". J. Neural Transm. (Vienna) 124 (Suppl 1): 3–26. July 2016. doi:10.1007/s00702-016-1593-7. PMID 27400928.
- ↑ Jump up to: 19.0 19.1 19.2 "Practice guideline update summary: Mild cognitive impairment – Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology". Neurology. Special article 90 (3): 126–135. January 2018. doi:10.1212/WNL.0000000000004826. PMID 29282327.
- ↑ Jump up to: 20.0 20.1 20.2 "Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments". Front. Psychiatry 6: 175. February 2016. doi:10.3389/fpsyt.2015.00175. PMID 26903885.
- ↑ Jump up to: 21.0 21.1 21.2 21.3 21.4 21.5 "Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis". Neurosci Biobehav Rev 37 (8): 1622–1644. September 2013. doi:10.1016/j.neubiorev.2013.06.011. PMID 23806439.
- ↑ Jump up to: 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 22.11 22.12 22.13 22.14 22.15 22.16 "Natural rewards, neuroplasticity, and non-drug addictions". Neuropharmacology 61 (7): 1109–1122. December 2011. doi:10.1016/j.neuropharm.2011.03.010. PMID 21459101.
- ↑ Jump up to: 23.0 23.1 23.2 "Exercise-based treatments for substance use disorders: evidence, theory, and practicality". Am J Drug Alcohol Abuse 41 (1): 7–15. 2015. doi:10.3109/00952990.2014.976708. PMID 25397661.
- ↑ Jump up to: 24.0 24.1 24.2 "The effect of exercise interventions on cognitive outcome in Alzheimer's disease: a systematic review". Int Psychogeriatr 26 (1): 9–18. January 2014. doi:10.1017/S1041610213001385. PMID 23962667.
- ↑ "Physiotherapy versus placebo or no intervention in Parkinson's disease". Cochrane Database Syst Rev 9 (9): CD002817. September 2013. doi:10.1002/14651858.CD002817.pub4. PMID 24018704.
- ↑ Jump up to: 26.0 26.1 "Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies". BMC Public Health 14: 510. May 2014. doi:10.1186/1471-2458-14-510. PMID 24885250.
- ↑ Jump up to: 27.0 27.1 Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. 2009. pp. 5, 351. ISBN 9780071481274.
- ↑ "Chapter 8:Atypical Neurotransmitters". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. 2009. pp. 199, 215. ISBN 9780071481274.
- ↑ Jump up to: 29.0 29.1 29.2 "A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor". J Psychiatr Res 60C: 56–64. October 2014. doi:10.1016/j.jpsychires.2014.10.003. PMID 25455510.
- ↑ Jump up to: 30.0 30.1 30.2 "Cerebral hemodynamics of the aging brain: risk of Alzheimer disease and benefit of aerobic exercise". Front Physiol 5: 6. January 2014. doi:10.3389/fphys.2014.00006. PMID 24478719.
- ↑ Jump up to: 31.0 31.1 31.2 "At least eighty percent of brain grey matter is modifiable by physical activity: A review study". Behavioural Brain Research 332: 204–217. June 2017. doi:10.1016/j.bbr.2017.06.002. PMID 28600001.
- ↑ Jump up to: 32.0 32.1 32.2 "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. 2009. pp. 147–148, 154–157. ISBN 9780071481274.
- ↑ "Effect of aerobic exercise on cognition, academic achievement, and psychosocial function in children: a systematic review of randomized control trials". Prev Chronic Dis 10: E174. 2013. doi:10.5888/pcd10.130010. PMID 24157077.
- ↑ "Physical activity and cognitive function in individuals over 60 years of age: a systematic review". Clin Interv Aging 9: 661–682. 2014. doi:10.2147/CIA.S55520. PMID 24748784.
- ↑ Jump up to: 35.0 35.1 "Mind over matter—what do we know about neuroplasticity in adults?". Int Psychogeriatr 26 (6): 891–909. June 2014. doi:10.1017/S1041610213002482. PMID 24382194.
- ↑ "Physical activity and memory functions: an interventional study". Neurobiol. Aging 32 (7): 1304–19. July 2011. doi:10.1016/j.neurobiolaging.2009.08.001. PMID 19716631.
- ↑ Jump up to: 37.0 37.1 37.2 "Exercise training increases size of hippocampus and improves memory". Proc. Natl. Acad. Sci. U.S.A. 108 (7): 3017–3022. February 2011. doi:10.1073/pnas.1015950108. PMID 21282661. Bibcode: 2011PNAS..108.3017E.
- ↑ Jump up to: 38.0 38.1 38.2 38.3 38.4 38.5 38.6 "Chapter 13: Higher Cognitive Function and Behavioral Control". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. 2009. pp. 313–321. ISBN 9780071481274.
- ↑ Jump up to: 39.0 39.1 39.2 Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. 2009. pp. 148, 324–328, 438. ISBN 9780071481274.
- ↑ "Cerebellar Transcranial Direct Current Stimulation (ctDCS): A Novel Approach to Understanding Cerebellar Function in Health and Disease". Neuroscientist 22 (1): 83–97. 2014. doi:10.1177/1073858414559409. PMID 25406224.
- ↑ Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. 2009. pp. 147, 266, 376. ISBN 9780071481274.
- ↑ "Multisensory maps in parietal cortex". Curr. Opin. Neurobiol. 24 (1): 39–46. 2014. doi:10.1016/j.conb.2013.08.014. PMID 24492077.
- ↑ "Chapter 13: Higher Cognitive Function and Behavioral Control". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. 2009. p. 315. ISBN 9780071481274.
- ↑ Jump up to: 44.0 44.1 44.2 "Effects of acute bouts of physical activity on children's attention: a systematic review of the literature". SpringerPlus 3: 410. 2014. doi:10.1186/2193-1801-3-410. PMID 25133092.
- ↑ "High-intensity training enhances executive function in children in a randomized, placebo-controlled trial". eLife 6:e25062. 2017. doi:10.7554/eLife.25062. PMID 28825973.
- ↑ Jump up to: 46.0 46.1 "Prescription Stimulants' Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis". J Cogn Neurosci 27 (6): 1–21. 2015. doi:10.1162/jocn_a_00776. PMID 25591060. https://repository.upenn.edu/neuroethics_pubs/130.
- ↑ "Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis". British Journal of Sports Medicine 52 (3): 154–160. February 2018. doi:10.1136/bjsports-2016-096587. PMID 28438770.
- ↑ Jump up to: 48.0 48.1 48.2 48.3 48.4 48.5 48.6 "Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling". Front Cell Neurosci 8: 170. 2014. doi:10.3389/fncel.2014.00170. PMID 24999318.
- ↑ Jump up to: 49.0 49.1 49.2 "Organ-Specific Physiological Responses to Acute Physical Exercise and Long-Term Training in Humans". Physiology 29 (6): 421–436. November 2014. doi:10.1152/physiol.00067.2013. PMID 25362636.
- ↑ "Effects of exercise and physical activity on anxiety". Frontiers in Psychiatry 4: 27. 2013. doi:10.3389/fpsyt.2013.00027. PMID 23630504.
- ↑ Jump up to: 51.0 51.1 51.2 51.3 "Toward a comprehensive neurobiology of IGF-I". Dev Neurobiol 70 (5): 384–96. 2010. doi:10.1002/dneu.20778. PMID 20186710.
- ↑ Jump up to: 52.0 52.1 52.2 "Role of the growth hormone/insulin-like growth factor 1 axis in neurogenesis". Endocr Dev. Endocrine Development 17: 63–76. 2010. doi:10.1159/000262529. ISBN 978-3-8055-9302-1. PMID 19955757.
- ↑ Jump up to: 53.0 53.1 53.2 Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. 2009. pp. 221, 412. ISBN 9780071481274.
- ↑ "IGF-I/IGFBP system: metabolism outline and physical exercise". J. Endocrinol. Invest. 35 (7): 699–707. July 2012. doi:10.3275/8456. PMID 22714057.
- ↑ Jump up to: 55.0 55.1 "Aging and brain rejuvenation as systemic events". J. Neurochem. 132 (1): 5–19. 2015. doi:10.1111/jnc.12969. PMID 25327899.
- ↑ "The hormone irisin is found to confer benefits of exercise on cognitive function" (in en). medicalxpress.com. https://medicalxpress.com/news/2021-08-hormone-irisin-confer-benefits-cognitive.html.
- ↑ "How Exercise May Help Keep Our Memory Sharp". The New York Times. 25 August 2021. https://www.nytimes.com/2021/08/25/well/move/exercise-brain-memory-benefits.html.
- ↑ "Exercise hormone irisin is a critical regulator of cognitive function". Nature Metabolism 3 (8): 1058–1070. August 2021. doi:10.1038/s42255-021-00438-z. PMID 34417591.
- ↑ "Progress and Challenges in the Biology of FNDC5 and Irisin". Endocrine Reviews 42 (4): 436–456. July 2021. doi:10.1210/endrev/bnab003. PMID 33493316.
- ↑ Jump up to: 60.0 60.1 "Acute Exercise Improves Prefrontal Cortex but not Hippocampal Function in Healthy Adults". Journal of the International Neuropsychological Society 21 (10): 791–801. November 2015. doi:10.1017/S135561771500106X. PMID 26581791.
- ↑ Jump up to: 61.0 61.1 "Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: a meta-analytical investigation". Brain and Cognition 80 (3): 338–351. December 2012. doi:10.1016/j.bandc.2012.09.001. PMID 23064033.
- ↑ "Electroencephalographic evidence for improved visual working memory performance during standing and exercise". British Journal of Psychology 110 (2): 400–427. May 2019. doi:10.1111/bjop.12352. PMID 30311188.
- ↑ Dodwell, Gordon; Liesefeld, Heinrich R.; Conci, Markus; Müller, Hermann J.; Töllner, Thomas (December 2021). "EEG evidence for enhanced attentional performance during moderate-intensity exercise" (in en). Psychophysiology 58 (12): e13923. doi:10.1111/psyp.13923. ISSN 0048-5772. PMID 34370887. https://onlinelibrary.wiley.com/doi/10.1111/psyp.13923.
- ↑ "[Levels of beta-endorphin in response to exercise and overtraining]" (in pt). Arq Bras Endocrinol Metabol 52 (4): 589–598. June 2008. doi:10.1590/S0004-27302008000400004. PMID 18604371.
- ↑ "The runner's high: opioidergic mechanisms in the human brain". Cereb. Cortex 18 (11): 2523–2531. 2008. doi:10.1093/cercor/bhn013. PMID 18296435. "The runner's high describes an euphoric state resulting from long-distance running.".
- ↑ Jump up to: 66.0 66.1 66.2 "Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the 'runner's high'". J. Exp. Biol. 215 (Pt 8): 1331–1336. 2012. doi:10.1242/jeb.063677. PMID 22442371.
- ↑ "Rowers' high: behavioural synchrony is correlated with elevated pain thresholds". Biol. Lett. 6 (1): 106–108. 2010. doi:10.1098/rsbl.2009.0670. PMID 19755532.
- ↑ Jump up to: 68.0 68.1 68.2 68.3 68.4 "Phenylethylamine, a possible link to the antidepressant effects of exercise?". Br J Sports Med 35 (5): 342–343. 2001. doi:10.1136/bjsm.35.5.342. PMID 11579070.
- ↑ Jump up to: 69.0 69.1 69.2 69.3 69.4 "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. 2005. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ↑ Jump up to: 70.0 70.1 70.2 70.3 70.4 "The potential of trace amines and their receptors for treating neurological and psychiatric diseases". Rev Recent Clin Trials 2 (1): 3–19. 2007. doi:10.2174/157488707779318107. PMID 18473983.
- ↑ Jump up to: 71.0 71.1 71.2 71.3 71.4 "Effects of exercise and physical activity on depression". Ir J Med Sci 180 (2): 319–325. 2011. doi:10.1007/s11845-010-0633-9. PMID 21076975.
- ↑ Jump up to: 72.0 72.1 72.2 72.3 72.4 "Physical activity and the endocannabinoid system: an overview". Cell. Mol. Life Sci. 71 (14): 2681–2698. 2014. doi:10.1007/s00018-014-1575-6. PMID 24526057.
- ↑ "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. May 2005. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ↑ "The endogenous substrates of brain CYP2D". Eur. J. Pharmacol. 724: 211–218. February 2014. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ↑ "Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges". Pharmacology & Therapeutics 180: 161–180. December 2017. doi:10.1016/j.pharmthera.2017.07.002. PMID 28723415.
- ↑ Jump up to: 76.0 76.1 "The vascular effects of trace amines and amphetamines". Pharmacol. Ther. 125 (3): 363–375. March 2010. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ↑ "Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans". Psychoneuroendocrinology 126: 105173. April 2021. doi:10.1016/j.psyneuen.2021.105173. PMID 33582575.
- ↑ Jump up to: 78.0 78.1 78.2 78.3 "Chapter 14: Mood and Emotion". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. 2009. pp. 350–359. ISBN 9780071481274.
- ↑ Jump up to: 79.0 79.1 79.2 79.3 79.4 "Neuroendocrine alterations in the exercising human: implications for energy homeostasis". Metab. Clin. Exp. 62 (7): 911–921. July 2013. doi:10.1016/j.metabol.2013.01.016. PMID 23415825.
- ↑ Jump up to: 80.0 80.1 80.2 80.3 "Hormones as 'difference makers' in cognitive and socioemotional aging processes". Front Psychol 5: 1595. January 2015. doi:10.3389/fpsyg.2014.01595. PMID 25657633.
- ↑ Jump up to: 81.0 81.1 "Exercise and physical activity in mental disorders: clinical and experimental evidence". J Prev Med Public Health 46 (Suppl 1): S12–521. January 2013. doi:10.3961/jpmph.2013.46.S.S12. PMID 23412549.
- ↑ "Chapter 5: Excitatory and Inhibitory Amino Acids". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. 2009. pp. 117–130. ISBN 9780071481274.
- ↑ Jump up to: 83.0 83.1 "(In)activity-related neuroplasticity in brainstem control of sympathetic outflow: unraveling underlying molecular, cellular, and anatomical mechanisms". Am. J. Physiol. Heart Circ. Physiol. 309 (2): H235–43. May 2015. doi:10.1152/ajpheart.00929.2014. PMID 25957223.
- ↑ "Exerkines in health, resilience and disease". Nature Reviews. Endocrinology 18 (5): 273–289. May 2022. doi:10.1038/s41574-022-00641-2. PMID 35304603.
- ↑ Strong, William B.; Malina, Robert M.; Blimkie, Cameron J.R. et al. (June 2005). "Evidence Based Physical Activity for School-age Youth". The Journal of Pediatrics 146 (6): 732–737. doi:10.1016/j.jpeds.2005.01.055. ISSN 0022-3476. PMID 15973308. http://dx.doi.org/10.1016/j.jpeds.2005.01.055.
- ↑ Physical activity levels among children aged 9-13 years: United States, 2002.. 2002. doi:10.1037/e303072004-001. http://dx.doi.org/10.1037/e303072004-001. Retrieved 2023-12-08.
- ↑ Ebbeling, Cara B; Pawlak, Dorota B; Ludwig, David S (August 2002). "Childhood obesity: public-health crisis, common sense cure". The Lancet 360 (9331): 473–482. doi:10.1016/s0140-6736(02)09678-2. ISSN 0140-6736. PMID 12241736. http://dx.doi.org/10.1016/s0140-6736(02)09678-2.
- ↑ Ward, Dianne Stanton; Saunders, Ruth P.; Pate, Russell R. (2007). Physical Activity Interventions in Children and Adolescents. doi:10.5040/9781492596868. ISBN 9781492596868. http://dx.doi.org/10.5040/9781492596868.
- ↑ van Sluijs, Esther M F; McMinn, Alison M; Griffin, Simon J (2007-09-20). "Effectiveness of interventions to promote physical activity in children and adolescents: systematic review of controlled trials". BMJ 335 (7622): 703. doi:10.1136/bmj.39320.843947.be. ISSN 0959-8138. PMID 17884863.
- ↑ Pate, Russell R.; Trost, Stewart G.; Mullis, Rebecca; Sallis, James F.; Wechsler, Howell; Brown, David R. (August 2000). "Community Interventions to Promote Proper Nutrition and Physical Activity among Youth". Preventive Medicine 31 (2): S138–S149. doi:10.1006/pmed.2000.0632. ISSN 0091-7435. http://dx.doi.org/10.1006/pmed.2000.0632.
- ↑ Stone, Elaine J; McKenzie, Thomas L; Welk, Gregory J; Booth, Michael L (November 1998). "Effects of physical activity interventions in youth". American Journal of Preventive Medicine 15 (4): 298–315. doi:10.1016/s0749-3797(98)00082-8. ISSN 0749-3797. PMID 9838974. http://dx.doi.org/10.1016/s0749-3797(98)00082-8.
- ↑ Jump up to: 92.0 92.1 92.2 "The Relationship between Physical Activity and Cognition in Children: A Meta-Analysis". Pediatric Exercise Science 15 (3): 243–256. August 2003. doi:10.1123/pes.15.3.243.
- ↑ "Effects of Physical Activity on Children's Executive Function: Contributions of Experimental Research on Aerobic Exercise". Developmental Review 30 (4): 331–551. December 2010. doi:10.1016/j.dr.2010.08.001. PMID 21818169.
- ↑ Jump up to: 94.0 94.1 "Be smart, exercise your heart: exercise effects on brain and cognition". Nature Reviews. Neuroscience 9 (1): 58–65. January 2008. doi:10.1038/nrn2298. PMID 18094706.
- ↑ "Effect of physical education and activity levels on academic achievement in children". Medicine and Science in Sports and Exercise 38 (8): 1515–1519. August 2006. doi:10.1249/01.mss.0000227537.13175.1b. PMID 16888468.
- ↑ "Aerobic fitness and executive control of relational memory in preadolescent children". Medicine and Science in Sports and Exercise 43 (2): 344–349. February 2011. doi:10.1249/MSS.0b013e3181e9af48. PMID 20508533.
- ↑ "Protection from genetic diathesis in attention-deficit/hyperactivity disorder: possible complementary roles of exercise". J. Am. Acad. Child Adolesc. Psychiatry 52 (9): 900–910. September 2013. doi:10.1016/j.jaac.2013.05.018. PMID 23972692.
- ↑ Jump up to: 98.0 98.1 "Physical activity interventions for people with mental illness: a systematic review and meta-analysis". J Clin Psychiatry 75 (9): 964–974. 2014. doi:10.4088/JCP.13r08765. PMID 24813261.
- ↑ Jump up to: 99.0 99.1 "Exercise for depression". Cochrane Database Syst. Rev. 2013 (9): CD004366. September 2013. doi:10.1002/14651858.CD004366.pub6. PMID 24026850.
- ↑ "Running is rewarding and antidepressive". Physiol. Behav. 92 (1–2): 136–140. 2007. doi:10.1016/j.physbeh.2007.05.015. PMID 17561174.
- ↑ "Yoga for prenatal depression: a systematic review and meta-analysis". BMC Psychiatry 15: 14. February 2015. doi:10.1186/s12888-015-0393-1. PMID 25652267.
- ↑ "Comparative effectiveness of three exercise types to treat clinical depression in older adults: A systematic review and network meta-analysis of randomised controlled trials". Ageing Research Reviews 58: 100999. 2020. doi:10.1016/j.arr.2019.100999. PMID 31837462. http://researchonline.federation.edu.au/vital/access/HandleResolver/1959.17/172086.
- ↑ "Somatization misattributed to non-pathological vaginal discharge". Journal of Psychosomatic Research 37 (6): 575–579. September 1993. doi:10.1016/0022-3999(93)90051-G. PMID 8410743.
- ↑ "Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study". Lancet 376 (9735): 112–123. July 2010. doi:10.1016/s0140-6736(10)60834-3. PMID 20561675.
- ↑ "Physical activity and stroke risk: a meta-analysis". Stroke 34 (10): 2475–2481. October 2003. doi:10.1161/01.STR.0000091843.02517.9D. PMID 14500932.
- ↑ "Pre-stroke physical activity in relation to post-stroke outcomes - linked to the International Classification of Functioning, Disability and Health (ICF): A scoping review". Journal of Rehabilitation Medicine 54: jrm00251. January 2022. doi:10.2340/jrm.v53.51. PMID 34904691.
- ↑ "Exercise-induced overexpression of angiogenic factors and reduction of ischemia/reperfusion injury in stroke". Current Neurovascular Research 1 (5): 411–420. December 2004. doi:10.2174/1567202043361875. PMID 16181089.
- ↑ "High intensity exercise preconditioning provides differential protection against brain injury following experimental stroke". Life Sciences 207: 30–35. August 2018. doi:10.1016/j.lfs.2018.03.007. PMID 29522768.
- ↑ "Treadmill exercise promotes angiogenesis in the ischemic penumbra of rat brains through caveolin-1/VEGF signaling pathways". Brain Research 1585: 83–90. October 2014. doi:10.1016/j.brainres.2014.08.032. PMID 25148708.
- ↑ "Mechanisms of stroke protection by physical activity". Annals of Neurology 54 (5): 582–590. November 2003. doi:10.1002/ana.10722. PMID 14595647.
- ↑ "Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow". Circulation Research 99 (10): 1132–1140. November 2006. doi:10.1161/01.RES.0000250175.14861.77. PMID 17038638.
- ↑ "Short-Term Acute Exercise Preconditioning Reduces Neurovascular Injury After Stroke Through Induced eNOS Activation". Translational Stroke Research 11 (4): 851–860. August 2020. doi:10.1007/s12975-019-00767-y. PMID 31858409.
- ↑ "HIF1 and oxygen sensing in the brain". Nature Reviews. Neuroscience 5 (6): 437–448. June 2004. doi:10.1038/nrn1408. PMID 15152194.
- ↑ "Mechanisms of neuronal damage and neuroprotection underlying ischemia/reperfusion injury after physical exercise". Current Drug Targets 13 (2): 247–262. February 2012. doi:10.2174/138945012799201658. PMID 22204323.
- ↑ "Exercise preconditioning reduces ischemia reperfusion-induced focal cerebral infarct volume through up-regulating the expression of HIF-1α". Pakistan Journal of Pharmaceutical Sciences 28 (2 Suppl): 791–798. March 2015. PMID 25796156. https://pubmed.ncbi.nlm.nih.gov/25796156.
- ↑ "Pre-ischemic treadmill training affects glutamate and gamma aminobutyric acid levels in the striatal dialysate of a rat model of cerebral ischemia". Life Sciences 84 (15–16): 505–511. April 2009. doi:10.1016/j.lfs.2009.01.015. PMID 19302809.
- ↑ "Pre-ischemic treadmill training induces tolerance to brain ischemia: involvement of glutamate and ERK1/2". Molecules 15 (8): 5246–5257. August 2010. doi:10.3390/molecules15085246. PMID 20714296.
- ↑ "Pre-ischemic treadmill training for prevention of ischemic brain injury via regulation of glutamate and its transporter GLT-1". International Journal of Molecular Sciences 13 (8): 9447–9459. 2012-07-26. doi:10.3390/ijms13089447. PMID 22949807.
- ↑ "Pre-ischemic exercise reduces apoptosis in hippocampal CA3 cells after cerebral ischemia by modulation of the Bax/Bcl-2 proteins ratio and prevention of caspase-3 activation". The Journal of Physiological Sciences 65 (5): 435–443. September 2015. doi:10.1007/s12576-015-0382-7. PMID 26012958.
- ↑ "Associations of Prestroke Physical Activity With Stroke Severity and Mortality After Intracerebral Hemorrhage Compared With Ischemic Stroke". Neurology 99 (19): e2137–e2148. November 2022. doi:10.1212/WNL.0000000000201097. PMID 36344278.
- ↑ "Prestroke physical activity is associated with admission haematoma volume and the clinical outcome of intracerebral haemorrhage". Stroke and Vascular Neurology 8 (6): 511–520. May 2023. doi:10.1136/svn-2023-002316. PMID 37137521.
- ↑ "Mature Adult Mice With Exercise-Preconditioning Show Better Recovery After Intracerebral Hemorrhage". Stroke 52 (5): 1861–1865. May 2021. doi:10.1161/STROKEAHA.120.032201. PMID 33840224.
- ↑ "Self-reported long-term needs after stroke". Stroke 42 (5): 1398–1403. May 2011. doi:10.1161/STROKEAHA.110.598839. PMID 21441153.
- ↑ "Physical Activity Trajectories and Functional Recovery After Acute Stroke Among Adults in Sweden". JAMA Network Open 6 (5): e2310919. May 2023. doi:10.1001/jamanetworkopen.2023.10919. PMID 37126346.
- ↑ "Associations Between Adherence to the Physical Activity and Exercise Program Applied in the LAST Study and Functional Recovery After Stroke". Archives of Physical Medicine and Rehabilitation 100 (12): 2251–2259. December 2019. doi:10.1016/j.apmr.2019.04.023. PMID 31374191.
- ↑ "Association of Levels of Physical Activity With Risk of Parkinson Disease: A Systematic Review and Meta-analysis". JAMA Network Open 1 (5): e182421. September 2018. doi:10.1001/jamanetworkopen.2018.2421. PMID 30646166.
- ↑ Mak, Margaret K.; Wong-Yu, Irene S.; Shen, Xia; Chung, Chloe L. (November 2017). "Long-term effects of exercise and physical therapy in people with Parkinson disease" (in en). Nature Reviews Neurology 13 (11): 689–703. doi:10.1038/nrneurol.2017.128. ISSN 1759-4766. PMID 29027544. https://www.nature.com/articles/nrneurol.2017.128.
- ↑ Jump up to: 128.0 128.1 Ernst, Moritz; Folkerts, Ann-Kristin; Gollan, Romina et al. (2023-01-05). "Physical exercise for people with Parkinson's disease: a systematic review and network meta-analysis". The Cochrane Database of Systematic Reviews 1 (1): CD013856. doi:10.1002/14651858.CD013856.pub2. ISSN 1469-493X. PMID 36602886.
 |