Biology:Receptor tyrosine kinase
| receptor protein-tyrosine kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC number | 2.7.10.1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Pkinase_Tyr | ||||||||
| Pfam | PF07714 | ||||||||
| OPM superfamily | 186 | ||||||||
| OPM protein | 2k1k | ||||||||
| Membranome | 3 | ||||||||
| |||||||||
Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase proteins.[1] Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer.[2] Mutations in receptor tyrosine kinases lead to activation of a series of signalling cascades which have numerous effects on protein expression.[3] Receptor tyrosine kinases are part of the larger family of protein tyrosine kinases, encompassing the receptor tyrosine kinase proteins which contain a transmembrane domain, as well as the non-receptor tyrosine kinases which do not possess transmembrane domains.[4]
History
The first RTKs to be discovered were the EGF and NGF receptors in the 1960s, but the classification of receptor tyrosine kinases was not developed until the 1970s.[5]
Classes
Approximately 20 different RTK classes have been identified.[6]
- RTK class I (EGF receptor family) (ErbB family)
- RTK class II (Insulin receptor family)
- RTK class III (PDGF receptor family)
- RTK class IV (VEGF receptors family)
- RTK class V (FGF receptor family)
- RTK class VI (CCK receptor family)
- RTK class VII (NGF receptor family)
- RTK class VIII (HGF receptor family)
- RTK class IX (Eph receptor family)
- RTK class X (AXL receptor family)
- RTK class XI (TIE receptor family)
- RTK class XII (RYK receptor family)
- RTK class XIII (DDR receptor family)
- RTK class XIV (RET receptor family)
- RTK class XV (ROS receptor family)
- RTK class XVI (LTK receptor family)
- RTK class XVII (ROR receptor family)
- RTK class XVIII (MuSK receptor family)
- RTK class XIX (LMR receptor)
- RTK class XX (Undetermined)
Structure
Most RTKs are single subunit receptors but some exist as multimeric complexes, e.g., the insulin receptor that forms disulfide linked dimers in the presence of hormone (insulin); moreover, ligand binding to the extracellular domain induces formation of receptor dimers.[7] Each monomer has a single hydrophobic transmembrane-spanning domain composed of 25 to 38 amino acids, an extracellular N terminal region, and an intracellular C terminal region.[8] The extracellular N terminal region exhibits a variety of conserved elements including immunoglobulin (Ig)-like or epidermal growth factor (EGF)-like domains, fibronectin type III repeats, or cysteine-rich regions that are characteristic for each subfamily of RTKs; these domains contain primarily a ligand-binding site, which binds extracellular ligands, e.g., a particular growth factor or hormone.[2] The intracellular C terminal region displays the highest level of conservation and comprises catalytic domains responsible for the kinase activity of these receptors, which catalyses receptor autophosphorylation and tyrosine phosphorylation of RTK substrates.[2]
Kinase activity
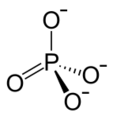
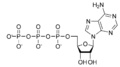
A kinase is a type of enzyme that transfers phosphate groups (see below) from high-energy donor molecules, such as ATP (see below) to specific target molecules (substrates); the process is termed phosphorylation. The opposite, an enzyme that removes phosphate groups from targets, is known as a phosphatase. Kinase enzymes that specifically phosphorylate tyrosine amino acids are termed tyrosine kinases.
When a growth factor binds to the extracellular domain of a RTK, its dimerization is triggered with other adjacent RTKs. Dimerization leads to a rapid activation of the protein's cytoplasmic kinase domains, the first substrate for these domains being the receptor itself. The activated receptor as a result then becomes autophosphorylated on multiple specific intracellular tyrosine residues.
Signal transduction
Through diverse means, extracellular ligand binding will typically cause or stabilize receptor dimerization. This allows a tyrosine in the cytoplasmic portion of each receptor monomer to be trans-phosphorylated by its partner receptor, propagating a signal through the plasma membrane.[9] The phosphorylation of specific tyrosine residues within the activated receptor creates binding sites for Src homology 2 (SH2) domain- and phosphotyrosine binding (PTB) domain-containing proteins.[10][11] Specific proteins containing these domains include Src and phospholipase Cγ. Phosphorylation and activation of these two proteins on receptor binding lead to the initiation of signal transduction pathways. Other proteins that interact with the activated receptor act as adaptor proteins and have no intrinsic enzymatic activity of their own. These adaptor proteins link RTK activation to downstream signal transduction pathways, such as the MAP kinase signalling cascade.[2] An example of a vital signal transduction pathway involves the tyrosine kinase receptor, c-met, which is required for the survival and proliferation of migrating myoblasts during myogenesis. A lack of c-met disrupts secondary myogenesis and—as in LBX1—prevents the formation of limb musculature. This local action of FGFs (Fibroblast Growth Factors) with their RTK receptors is classified as paracrine signalling. As RTK receptors phosphorylate multiple tyrosine residues, they can activate multiple signal transduction pathways.
Families
Epidermal growth factor receptor family
The ErbB protein family or epidermal growth factor receptor (EGFR) family is a family of four structurally related receptor tyrosine kinases. Insufficient ErbB signaling in humans is associated with the development of neurodegenerative diseases, such as multiple sclerosis and Alzheimer's disease.[12] In mice, loss of signaling by any member of the ErbB family results in embryonic lethality with defects in organs including the lungs, skin, heart, and brain. Excessive ErbB signaling is associated with the development of a wide variety of types of solid tumor. ErbB-1 and ErbB-2 are found in many human cancers and their excessive signaling may be critical factors in the development and malignancy of these tumors.[13]
Fibroblast growth factor receptor (FGFR) family
Fibroblast growth factors comprise the largest family of growth factor ligands at 23 members.[14] The natural alternate splicing of four fibroblast growth factor receptor (FGFR) genes results in the production of over 48 different isoforms of FGFR.[15] These isoforms vary in their ligand binding properties and kinase domains; however, all share a common extracellular region composed of three immunoglobulin (Ig)-like domains (D1-D3), and thus belong to the immunoglobulin superfamily.[16] Interactions with FGFs occur via FGFR domains D2 and D3. Each receptor can be activated by several FGFs. In many cases, the FGFs themselves can also activate more than one receptor. This is not the case with FGF-7, however, which can activate only FGFR2b.[15] A gene for a fifth FGFR protein, FGFR5, has also been identified. In contrast to FGFRs 1-4, it lacks a cytoplasmic tyrosine kinase domain, and one isoform, FGFR5γ, only contains the extracellular domains D1 and D2.[17]
Vascular endothelial growth factor receptor (VEGFR) family
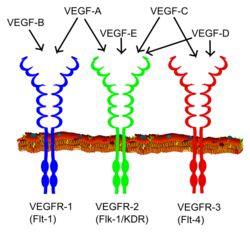
Vascular endothelial growth factor (VEGF) is one of the main inducers of endothelial cell proliferation and permeability of blood vessels. Two RTKs bind to VEGF at the cell surface, VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1).[18]
The VEGF receptors have an extracellular portion consisting of seven Ig-like domains so, like FGFRs, belong to the immunoglobulin superfamily. They also possess a single transmembrane spanning region and an intracellular portion containing a split tyrosine-kinase domain. VEGF-A binds to VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1). VEGFR-2 appears to mediate almost all of the known cellular responses to VEGF. The function of VEGFR-1 is less well defined, although it is thought to modulate VEGFR-2 signaling. Another function of VEGFR-1 may be to act as a dummy/decoy receptor, sequestering VEGF from VEGFR-2 binding (this appears to be particularly important during vasculogenesis in the embryo). A third receptor has been discovered (VEGFR-3); however, VEGF-A is not a ligand for this receptor. VEGFR-3 mediates lymphangiogenesis in response to VEGF-C and VEGF-D.
RET receptor family
The natural alternate splicing of the RET gene results in the production of 3 different isoforms of the protein RET. RET51, RET43, and RET9 contain 51, 43, and 9 amino acids in their C-terminal tail, respectively.[19] The biological roles of isoforms RET51 and RET9 are the most well studied in-vivo, as these are the most common isoforms in which RET occurs.
RET is the receptor for members of the glial cell line-derived neurotrophic factor (GDNF) family of extracellular signalling molecules or ligands (GFLs).[20]
In order to activate RET, first GFLs must form a complex with a glycosylphosphatidylinositol (GPI)-anchored co-receptor. The co-receptors themselves are classified as members of the GDNF receptor-α (GFRα) protein family. Different members of the GFRα family (GFRα1-GFRα4) exhibit a specific binding activity for a specific GFLs.[21] Upon GFL-GFRα complex formation, the complex then brings together two molecules of RET, triggering trans-autophosphorylation of specific tyrosine residues within the tyrosine kinase domain of each RET molecule. Phosphorylation of these tyrosines then initiates intracellular signal transduction processes.[22]
Eph receptor family
Ephrin receptors are the largest subfamily of RTKs.
Discoidin domain receptor (DDR) family
The DDRs are unique RTKs in that they bind to collagens rather than soluble growth factors.[23]
Regulation
The receptor tyrosine kinase (RTK) pathway is carefully regulated by a variety of positive and negative feedback loops.[24] Because RTKs coordinate a wide variety of cellular functions such as cell proliferation and differentiation, they must be regulated to prevent severe abnormalities in cellular functioning such as cancer and fibrosis.[25]
Protein tyrosine phosphatases
Protein Tyrosine Phosphatase (PTPs) are a group of enzymes that possess a catalytic domain with phosphotyrosine-specific phosphohydrolase activity. PTPs are capable of modifying the activity of receptor tyrosine kinases in both a positive and negative manner.[26] PTPs can dephosphorylate the activated phosphorylated tyrosine residues on the RTKs[27] which virtually leads to termination of the signal. Studies involving PTP1B, a widely known PTP involved in the regulation of the cell cycle and cytokine receptor signaling, has shown to dephosphorylate the epidermal growth factor receptor[28] and the insulin receptor.[29] Some PTPs, on the other hand, are cell surface receptors that play a positive role in cell signaling proliferation. Cd45, a cell surface glycoprotein, plays a critical role in antigen-stimulated dephosphorylation of specific phosphotyrosines that inhibit the Src pathway.[30]
Herstatin
Herstatin is an autoinhibitor of the ErbB family,[31] which binds to RTKs and blocks receptor dimerization and tyrosine phosphorylation.[27] CHO cells transfected with herstatin resulted in reduced receptor oligomerization, clonal growth and receptor tyrosine phosphorylation in response to EGF.[32]
Receptor endocytosis
Activated RTKs can undergo endocytosis resulting in down regulation of the receptor and eventually the signaling cascade.[3] The molecular mechanism involves the engulfing of the RTK by a clathrin-mediated endocytosis, leading to intracellular degradation.[3]
Drug therapy
RTKs have become an attractive target for drug therapy due to their implication in a variety of cellular abnormalities such as cancer, degenerative diseases and cardiovascular diseases. The United States Food and Drug Administration (FDA) has approved several anti-cancer drugs caused by activated RTKs. Drugs have been developed to target the extracellular domain or the catalytic domain, thus inhibiting ligand binding, receptor oligomerization.[33] Herceptin, a monoclonal antibody that is capable of binding to the extracellular domain of RTKs, has been used to treat HER2 overexpression in breast cancer.[34]
| Small Molecule | Target | Disease | Approval Year |
|---|---|---|---|
| Imatinib (Gleevec) | PDGFR, KIT, Abl, Arg | CML, GIST | 2001 |
| Gefitinib (Iressa) | EGFR | Esophageal cancer, Glioma | 2003 |
| Erlotinib (Tarceva) | EGFR | Esophageal cancer, Glioma | 2004 |
| Sorafenib (Nexavar) | Raf, VEGFR, PDGFR, Flt3, KIT | Renal cell carcinoma | 2005 |
| Sunitinib (Sutent) | KIT, VEGFR, PDGFR, Flt3 | Renal cell carcinoma, GIST, Endocrine pancreatic cancer | 2006 |
| Dasatinib (Sprycel) | Abl, Arg, KIT, PDGFR, Src | Imatinib-resistant CML | 2007 |
| Nilotinib (Tasigna) | Abl, Arg, KIT, PDGFR | Imatinib-resistant CML | 2007 |
| Lapatinib (Tykerb) | EGFR, ErbB2 | Mammary carcinoma | 2007 |
| Trastuzumab (Herceptin) | ErbB2 | Mammary carcinoma | 1998 |
| Cetuximab (Erbitux) | EGFR | Colorectal cancer, Head and neck cancer | 2004 |
| Bevacizumab (Avastin) | VEGF | Lung cancer, Colorectal cancer | 2004 |
| Panitumumab (Vectibix) | EGFR | Colorectal cancer | 2006 |
+ Table adapted from "Cell signalling by receptor-tyrosine kinases," by Lemmon and Schlessinger's, 2010. Cell, 141, p. 1117–1134.
See also
- Tyrosine kinase
- Insulin receptor
- Enzyme-linked receptor
- Tyrphostins
- Bcr-Abl tyrosine kinase inhibitors
References
- ↑ "The protein tyrosine kinase family of the human genome". Oncogene 19 (49): 5548–57. November 2000. doi:10.1038/sj.onc.1203957. PMID 11114734.
- ↑ 2.0 2.1 2.2 2.3 "Receptor tyrosine kinase signalling as a target for cancer intervention strategies". Endocrine-Related Cancer 8 (3): 161–73. September 2001. doi:10.1677/erc.0.0080161. PMID 11566607.
- ↑ 3.0 3.1 3.2 3.3 "Cell signaling by receptor tyrosine kinases". Cell 141 (7): 1117–34. June 2010. doi:10.1016/j.cell.2010.06.011. PMID 20602996.
- ↑ "Protein tyrosine kinase structure and function". Annual Review of Biochemistry 69: 373–98. 2000. doi:10.1146/annurev.biochem.69.1.373. PMID 10966463.
- ↑ Schlessinger, J. (3 March 2014). "Receptor Tyrosine Kinases: Legacy of the First Two Decades". Cold Spring Harbor Perspectives in Biology 6 (3): a008912. doi:10.1101/cshperspect.a008912. PMID 24591517.
- ↑ Ségaliny, Aude I.; Tellez-Gabriel, Marta; Heymann, Marie-Françoise; Heymann, Dominique (2015). "Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers". Journal of Bone Oncology 4 (1): 1–12. doi:10.1016/j.jbo.2015.01.001. PMID 26579483.
- ↑ Lodish (2003). Molecular cell biology (5th ed.). https://archive.org/details/molecularcellbio00harv.
- ↑ "Structural analysis of receptor tyrosine kinases". Progress in Biophysics and Molecular Biology 71 (3–4): 343–58. 1999. doi:10.1016/S0079-6107(98)00047-9. PMID 10354703.
- ↑ "Cell signaling by receptor tyrosine kinases". Cell 141 (7): 1117–34. June 2010. doi:10.1016/j.cell.2010.06.011. PMID 20602996.
- ↑ "Protein modules and signalling networks". Nature 373 (6515): 573–80. February 1995. doi:10.1038/373573a0. PMID 7531822. Bibcode: 1995Natur.373..573P.
- ↑ "The conservation pattern of short linear motifs is highly correlated with the function of interacting protein domains". BMC Genomics 9: 452. October 2008. doi:10.1186/1471-2164-9-452. PMID 18828911.
- ↑ "The EGF receptor family: spearheading a merger of signaling and therapeutics". Current Opinion in Cell Biology 19 (2): 124–34. April 2007. doi:10.1016/j.ceb.2007.02.008. PMID 17314037.
- ↑ "Structure of the extracellular region of HER3 reveals an interdomain tether". Science 297 (5585): 1330–3. August 2002. doi:10.1126/science.1074611. PMID 12154198. Bibcode: 2002Sci...297.1330C.
- ↑ "Fibroblast growth factors". Genome Biology 2 (3): REVIEWS3005. 2001. doi:10.1186/gb-2001-2-3-reviews3005. PMID 11276432.
- ↑ 15.0 15.1 "N-glycosylation of fibroblast growth factor receptor 1 regulates ligand and heparan sulfate co-receptor binding". The Journal of Biological Chemistry 281 (37): 27178–89. September 2006. doi:10.1074/jbc.M601248200. PMID 16829530.
- ↑ "Receptors for fibroblast growth factors". Immunology and Cell Biology 73 (6): 584–9. December 1995. doi:10.1038/icb.1995.92. PMID 8713482.
- ↑ "Identification of a new fibroblast growth factor receptor, FGFR5". Gene 271 (2): 171–82. June 2001. doi:10.1016/S0378-1119(01)00518-2. PMID 11418238.
- ↑ "The splice variants of vascular endothelial growth factor (VEGF) and their receptors". Journal of Cell Science 114 (Pt 5): 853–65. March 2001. doi:10.1242/jcs.114.5.853. PMID 11181169.
- ↑ "Characterization of RET proto-oncogene 3' splicing variants and polyadenylation sites: a novel C-terminus for RET". Oncogene 11 (10): 2039–45. November 1995. PMID 7478523.
- ↑ "The GDNF family ligands and receptors - implications for neural development". Current Opinion in Neurobiology 10 (1): 103–10. February 2000. doi:10.1016/S0959-4388(99)00048-3. PMID 10679429.
- ↑ "GDNF family neurotrophic factor signaling: four masters, one servant?". Molecular and Cellular Neurosciences 13 (5): 313–25. May 1999. doi:10.1006/mcne.1999.0754. PMID 10356294.
- ↑ "RET tyrosine kinase signaling in development and cancer". Cytokine & Growth Factor Reviews 16 (4–5): 441–67. 2005. doi:10.1016/j.cytogfr.2005.05.010. PMID 15982921.
- ↑ "Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling". The Journal of Biological Chemistry 288 (11): 7430–7. March 2013. doi:10.1074/jbc.R112.444158. PMID 23335507.
- ↑ "Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases". Trends in Cell Biology 11 (6): 258–66. June 2001. doi:10.1016/s0962-8924(01)01990-0. PMID 11356362.
- ↑ "Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B" (in en). The Journal of Biological Chemistry 278 (2): 739–44. January 2003. doi:10.1074/jbc.M210194200. PMID 12424235.
- ↑ "Complexity of receptor tyrosine kinase signal processing". Cold Spring Harbor Perspectives in Biology 5 (8): a009043. August 2013. doi:10.1101/cshperspect.a009043. PMID 23906711.
- ↑ 27.0 27.1 "Negative Regulation of Receptor Tyrosine Kinase (RTK) Signaling: A Developing Field". Biomarker Insights 2: 45–58. February 2007. doi:10.1177/117727190700200029. PMID 19662191.
- ↑ "Development of "substrate-trapping" mutants to identify physiological substrates of protein tyrosine phosphatases". Proceedings of the National Academy of Sciences of the United States of America 94 (5): 1680–5. March 1997. doi:10.1073/pnas.94.5.1680. PMID 9050838. Bibcode: 1997PNAS...94.1680F.
- ↑ "Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling". The Journal of Biological Chemistry 271 (33): 19810–6. August 1996. doi:10.1074/jbc.271.33.19810. PMID 8702689.
- ↑ "CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells". Immunological Reviews 228 (1): 288–311. March 2009. doi:10.1111/j.1600-065X.2008.00752.x. PMID 19290935.
- ↑ "Herstatin, an autoinhibitor of the human epidermal growth factor receptor 2 tyrosine kinase, modulates epidermal growth factor signaling pathways resulting in growth arrest". The Journal of Biological Chemistry 277 (23): 20618–24. 2002. doi:10.1074/jbc.M111359200. PMID 11934884.
- ↑ "Expression of herstatin, an autoinhibitor of HER-2/neu, inhibits transactivation of HER-3 by HER-2 and blocks EGF activation of the EGF receptor". Oncogene 20 (37): 5199–209. August 2001. doi:10.1038/sj.onc.1204555. PMID 11526509.
- ↑ "Targeting the EGFR signaling pathway in cancer therapy". Expert Opinion on Therapeutic Targets 16 (1): 15–31. January 2012. doi:10.1517/14728222.2011.648617. PMID 22239438.
- ↑ "HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review". British Journal of Cancer 90 (12): 2344–8. June 2004. doi:10.1038/sj.bjc.6601881. PMID 15150568.
External links
- Tyrosine+Kinase+Receptors at the US National Library of Medicine Medical Subject Headings (MeSH)
- EC 2.7.10.1
 |