Chemistry:Lithium polonide: Difference between revisions
From HandWiki
(linkage) |
StanislovAI (talk | contribs) (fix) |
||
| Line 1: | Line 1: | ||
{{Chembox | {{Chembox | ||
| ImageFile =CaF2_polyhedra.png | | ImageFile = CaF2_polyhedra.png | ||
| ImageCaption = Crystal structure of lithium polonide<br /><span style="color:#99CC00;background-color:#99CC00;">__</span> Li<sup>+</sup> <span style="color:#C0C0C0; background-color:#C0C0C0;">__</span> Po<sup>2-</sup> | | ImageCaption = Crystal structure of lithium polonide<br /><span style="color:#99CC00;background-color:#99CC00;">__</span> Li<sup>+</sup> <span style="color:#C0C0C0; background-color:#C0C0C0;">__</span> Po<sup>2-</sup> | ||
| | | PIN = Lithium polonide | ||
|Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| SMILES = [Li+].[Li+].[Po-2] | | SMILES = [Li+].[Li+].[Po-2] | ||
| StdInChI=1S/2Li.Po/q2*+1;-2 | | StdInChI=1S/2Li.Po/q2*+1;-2 | ||
}} | }} | ||
|Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| Formula = Li<sub>2</sub>Po | | Formula = Li<sub>2</sub>Po | ||
| MolarMass = 222.86 g/mol | | MolarMass = 222.86 g/mol | ||
| Appearance = greyish<ref name="Bagnall">{{cite book |title=Advances in Inorganic Chemistry and Radiochemistry |chapter=The Chemistry of Polonium |last1=Bagnall |first1=K. W. |year=1962 |publisher=[[Company:Academic Press|Academic Press]] |location=New York |isbn=9780120236046 |pages=197–230 |accessdate=June 17, 2012 |url=https://books.google.com/books?id=8qePsa3V8GQC}}</ref> | | Appearance = greyish<ref name="Bagnall">{{cite book |title=Advances in Inorganic Chemistry and Radiochemistry |chapter=The Chemistry of Polonium |last1=Bagnall |first1=K. W. |year=1962 |publisher=[[Company:Academic Press|Academic Press]] |location=New York |isbn=9780120236046 |pages=197–230 |accessdate=June 17, 2012 |url=https://books.google.com/books?id=8qePsa3V8GQC}}</ref> | ||
}} | }} | ||
| Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| Related_ref = | | Related_ref = | ||
| OtherAnions = [[Chemistry:Lithium oxide|Lithium oxide]]<br>[[Chemistry:Lithium sulfide|Lithium sulfide]]<br>[[Chemistry:Lithium selenide|Lithium selenide]]<br>[[Chemistry:Lithium telluride|Lithium telluride]] | | OtherAnions = [[Chemistry:Lithium oxide|Lithium oxide]]<br>[[Chemistry:Lithium sulfide|Lithium sulfide]]<br>[[Chemistry:Lithium selenide|Lithium selenide]]<br>[[Chemistry:Lithium telluride|Lithium telluride]] | ||
| OtherCations = | | OtherCations =[[Chemistry:Polonium hydride|Polonium hydride]]<br>[[Chemistry:Sodium polonide|Sodium polonide]]<br>[[Chemistry:Potassium polonide|Potassium polonide]] | ||
| OtherFunction = | | OtherFunction = | ||
| OtherFunction_label = | | OtherFunction_label = | ||
| Line 24: | Line 24: | ||
==Production== | ==Production== | ||
Lithium polonide may be produced from a redox reaction between aqueous | Lithium polonide may be produced from a redox reaction between aqueous [[Chemistry:Polonium hydride|polonium hydride]] and [[Chemistry:Lithium|lithium]] metal<ref name="G&E"/><ref name=Moyer/> or from an acid-base reaction of H<sub>2</sub>Po with strong lithium-containing bases: | ||
:H<sub>2</sub>Po + 2 Li → Li<sub>2</sub>Po + H<sub>2</sub> | :H<sub>2</sub>Po + 2 Li → Li<sub>2</sub>Po + H<sub>2</sub> | ||
| Line 36: | Line 36: | ||
{{reflist}} | {{reflist}} | ||
{{Lithium compounds}} | {{Lithium compounds}} | ||
{{Polonium compounds}} | |||
[[Category:Lithium compounds]] | [[Category:Lithium compounds]] | ||
{{Sourceattribution|Lithium polonide}} | {{Sourceattribution|Lithium polonide}} | ||
Latest revision as of 16:28, 15 July 2025
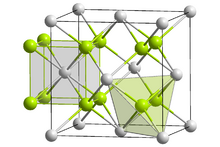 Crystal structure of lithium polonide
__ Li+ __ Po2- | |
| Names | |
|---|---|
| Preferred IUPAC name
Lithium polonide | |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| Li2Po | |
| Molar mass | 222.86 g/mol |
| Appearance | greyish[1] |
| Related compounds | |
Other anions
|
Lithium oxide Lithium sulfide Lithium selenide Lithium telluride |
Other cations
|
Polonium hydride Sodium polonide Potassium polonide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lithium polonide is a chemical compound with the formula Li2Po. It is a polonide, a set of very chemically stable compounds of polonium.[2][3]
Production
Lithium polonide may be produced from a redox reaction between aqueous polonium hydride and lithium metal[2][3] or from an acid-base reaction of H2Po with strong lithium-containing bases:
- H2Po + 2 Li → Li2Po + H2
It may also be produced by heating lithium and polonium together at 300–400 °C.[1]
Crystal structure
Like sodium polonide, lithium polonide has the antifluorite structure.[2][3]
References
- ↑ 1.0 1.1 Bagnall, K. W. (1962). "The Chemistry of Polonium". Advances in Inorganic Chemistry and Radiochemistry. New York: Academic Press. pp. 197–230. ISBN 9780120236046. https://books.google.com/books?id=8qePsa3V8GQC. Retrieved June 17, 2012.
- ↑ 2.0 2.1 2.2 Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 899. ISBN 978-0-08-022057-4. https://books.google.com/books?id=OezvAAAAMAAJ&q=0-08-022057-6&dq=0-08-022057-6&source=bl&ots=m4tIRxdwSk&sig=XQTTjw5EN9n5z62JB3d0vaUEn0Y&hl=en&sa=X&ei=UoAWUN7-EM6ziQfyxIDoCQ&ved=0CD8Q6AEwBA.
- ↑ 3.0 3.1 3.2 Moyer, Harvey V. (1956), "Chemical Properties of Polonium", in Moyer, Harvey V., Polonium, Oak Ridge, Tenn.: United States Atomic Energy Commission, pp. 33–96, doi:10.2172/4367751, TID-5221, http://www.osti.gov/bridge/servlets/purl/4367751-nEJIbm/.
 |

