Chemistry:N,N-Diisopropylaminoethanol
| |||
| |||
|
| |||
| Names | |||
|---|---|---|---|
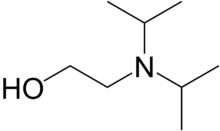
| Preferred IUPAC name
2-[Di(propan-2-yl)amino]ethan-1-ol | |||
| Other names
2-[Di(propan-2-yl)amino]ethanol
2-(Diisopropylamino)ethanol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1697955 | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| MeSH | 2-diisopropylaminoethanol | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2922 | ||
| |||
| |||
| Properties | |||
| C8H19NO | |||
| Molar mass | 145.246 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Ammoniacal | ||
| Density | 826 mg mL−1 | ||
| Melting point | −39.2 °C; −38.6 °F; 233.9 K | ||
| Boiling point | 190.1 °C; 374.1 °F; 463.2 K | ||
| log P | 1.476 | ||
| Vapor pressure | <100 Pa (at 20 °C) | ||
Refractive index (nD)
|
1.442 | ||
| Hazards | |||
| Safety data sheet | [1] | ||
| GHS pictograms |  
| ||
| H302, H311, H314, H331 | |||
| P261, P280, P305+351+338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 64 °C (147 °F; 337 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
| ||
| Related compounds | |||
Related alkanols
|
|||
Related compounds
|
Diethylhydroxylamine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
N,N-Diisopropylaminoethanol (DIPA) is a processor for production of various chemicals and also an intermediate in the production of the nerve agents VX and NX. [2] It is a colorless liquid, although aged samples can appear yellow.
Health effects
Inhalation and skin contact are expected to be the primary ways of occupational exposure to this chemical. Based on single exposure animal tests, it is considered to be slightly toxic if swallowed or inhaled, moderately toxic if absorbed through skin as well as being corrosive to eyes and skin.[1] Vapor may be irritating to the eyes and upper respiratory tract. Temporary and reversible visual disturbances characterized by mildly blurred vision, a blue-gray discolorization of sight (blue haze) or halo vision (appearance of a halo when looking at light sources) may also occur.[citation needed]
References
- ↑ 1.0 1.1 "SAFETY DATA SHEET 2--(Diisopropylamino)ethanol". MilliporeSigma. November 14, 2021. https://www.sigmaaldrich.com/US/en/sds/aldrich/471488.
- ↑ Suzuki, Osamu, ed (2005). Drugs and poisons in humans : a handbook of practical analysis (1. Aufl. ed.). Berlin [u.a.]: Springer. pp. 69–90. doi:10.1007/3-540-27579-7_9. ISBN 978-3-540-22277-4.
 |