Chemistry:Potassium octachlorodimolybdate

| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| K 4[Mo 2Cl 8] | |
| Molar mass | 631.89 g·mol−1 |
| Appearance | red crystals |
| Density | 2.54 g/cm3 |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium octachlorodimolybdate (systematically named potassium bis(tetrachloromolybdate)(Mo–Mo)(4−)) is an inorganic compound with the chemical formula K
4[Mo
2Cl
8]. It is known as a red-coloured, microcrystalline solid. The anion is of historic interest as one of the earliest illustrations of a quadruple bonding. The salt is usually obtained as the pink-coloured dihydrate.
The compound is prepared in two steps from molybdenum hexacarbonyl:[1][2]
- 2 Mo(CO)
6 + 4 CH
3CO
2H → (CH
3CO
2)
4Mo
2 + 2 H
2 + 12 CO - (CH
3CO
2)
4Mo
2 + 4 HCl + 4 KCl → K
4[Mo
2Cl
8] + 4 CH
3CO
2H
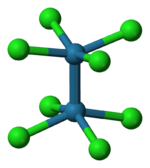
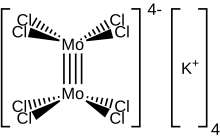
The reaction of the acetate with HCl was first described as providing trimolybdenum compounds,[3] but subsequent crystallographic analysis confirmed that the salt contains the [Cl
4Mo≣MoCl
4]4− anion, with D4h symmetry, in which the two Mo atoms are linked by a quadruple bond. Each Mo atom is bounded with four Cl−
ligands by a single bond. Each MoCl
4 group is an regular square pyramid, with an Mo atom at the apex, and four Cl atoms at the vertices of the square base of the pyramid. The Mo–Mo distance is 214 pm.[4]
References
- ↑ Brignole, A. B.; Cotton, F. A.; Dori, Z. (1972). "Rhenium and Molybdenum Compounds Containing Quadruple Bonds". Inorganic Syntheses. 13. 81–89. doi:10.1002/9780470132449.ch15. ISBN 978-0-470-13244-9.
- ↑ Girolami, G. S.; Rauchfuss, T. B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry. Mill Valley, CA: University Science Books. ISBN 978-0-935702-48-4.
- ↑ Allison, G. B.; Anderson, I. R.; Sheldon, J. C. (1967). "The Preparation of Halogenotrimolybdate(II) Compounds". Aust. J. Chem. 20 (5): 869–876. doi:10.1071/CH9670869.
- ↑ Brencic, Jurij V.; Cotton, F. Albert (1969). "Octachlorodimolybdate(II) Ion. Species with a Quadruple Metal–Metal Bond". Inorg. Chem. 8: 7–10. doi:10.1021/ic50071a002.
 |