Chemistry:Perchloromethyl mercaptan
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Trichloromethyl thiohypochlorite | |
| Other names
Trichloromethane sulfenyl chloride
Trichloromethyl sulfur chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1670 |
| |
| |
| Properties | |
| CCl4S | |
| Molar mass | 185.87 g·mol−1 |
| Appearance | Oily, yellow liquid |
| Odor | disagreeable, acrid odor |
| Density | 1.72 g/cm3 |
| Melting point | −44 °C (−47 °F; 229 K) |
| Boiling point | 147 to 148 °C (297 to 298 °F; 420 to 421 K) |
| Insoluble, reacts | |
| log P | 3.47 (estimated) |
| Vapor pressure | 0.4 kPa (at 20 °C) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H301, H311, H312, H314, H330, H335 | |
| P260, P261, P264, P270, P271, P280, P284, P301+310, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P320, P321, P322, P330, P361, P363, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
82.6 mg/kg (rat, oral)[2] |
LC50 (median concentration)
|
11 ppm (rat, 1 hr) 16 ppm (rat, 1 hr) 9 ppm (mouse, 3 hr) 38 ppm (mouse, 2 hr) 11 ppm (rat, 1 hr)[2] |
LCLo (lowest published)
|
388 ppm (human, 10 min) 46 ppm (mouse, 10 min)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.1 ppm (0.8 mg/m3)[1] |
REL (Recommended)
|
TWA 0.1 ppm (0.8 mg/m3)[1] |
IDLH (Immediate danger)
|
10 ppm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
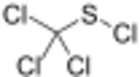
Perchloromethyl mercaptan is the organosulfur compound with the formula CCl3SCl. It is mainly used as an intermediate for the synthesis of dyes and fungicides (captan, folpet). It is a colorless oil, although commercial samples are yellowish. It is insoluble in water but soluble in organic solvents. It has a foul, unbearable, acrid odor. Perchloromethyl mercaptan is the original name. The systematic name is trichloromethanesulfenyl chloride, because the compound is a sulfenyl chloride, not a mercaptan.[3]
History
It was used as a chemical warfare agent by the French in the 1915 battle of Champagne. Shortly thereafter, wartime use was abandoned due to the clear warning properties, the decomposition in the presence of iron and steel, and the easy removal of the vapor by charcoal.[4]
Preparation
The method to prepare perchloromethyl mercaptan was first described by Rathke in 1873[3] and is still used. Carbon disulfide is chlorinated using an iodine catalyst. The following equations operate most efficiently at temperatures below about 30 °C
- CS2 + 3 Cl2 → CCl3SCl + SCl2
- 2 CS2 + 5 Cl2 → 2 CCl3SCl + S2Cl2
At higher temperatures, the chlorination gives carbon tetrachloride and additional sulfur chlorides.[5] The formation of byproducts can be suppressed by performing the reaction in the presence of diketones.[6] Another byproduct is thiophosgene. The more volatile byproducts such as carbon tetrachloride and sulfur dichloride can be removed by distillation. The separation of perchloromethyl mercaptan from S2Cl2 by distillation is challenging since their boiling points are very close. Another byproduct that forms is hexachloroethane. Innovations in the basic Rathke method have been reported.[6]
Reactivity
The compound slowly hydrolyzes:[6]
- CSCl4 + 2 H2O → CO2 + 4 HCl + 1⁄8 S8
The compound is corrosive to most metals. It reacts with iron, evolving carbon tetrachloride. Perchloromethyl mercaptan is oxidized by nitric acid to trichloromethanesulfonyl chloride (Cl3CSO2Cl), a white solid.[4]
Toxicity
| Animal | Oral | Inhalation | Intraperitoneal | Intravenous | Skin | Eyes |
| Rat | 82,6 mg/kg | 11 ppm/1h | 25 mg/kg | |||
| Mouse | 40 mg/kg | 296 g/m3/2h | 10 mg/kg | 56 mg/kg | ||
| Rabbit | 1410 mg/kg | |||||
| Guinea Pig | 500 μL/kg | 50 μg/24h[7] |
When it is heated or in a fire, it will emit toxic and corrosive gases. It is also very toxic by inhalation or skin absorption.[3]
At least two mechanisms could account for the toxicity of perchloromethyl mercaptan, as hypothesized by Althoff (1973). The first mechanism is a reaction between perchloromethyl mercaptan and biological functional groups such as hydroxyl, sulfhydryl, amino and carboxyl groups. This results in an inactivation of key enzymes. A second general pathway reaction is the hydrolysis to give hydrochloric acid.[3]
References
- ↑ Jump up to: 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0489". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0489.html.
- ↑ Jump up to: 2.0 2.1 2.2 "Perchloromethyl mercaptan". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/594423.html.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Committee on Acute Exposure Guideline Levels, Committee on Toxicology, National Research Council, (2011), Acute Exposure Guideline Levels for Selected Airborne Chemicals.
- ↑ Jump up to: 4.0 4.1 Sosnovsky, George "The chemistry of trichloromethanesulfenyl chloride" Chemical Reviews 1958, volume 58, 509-40. doi:10.1021/cr50021a003
- ↑ Manchiu D. S. Lay, Mitchell W. Sauerhoff And Donald R. Saunders "Carbon Disulfide" in Ullmann's Encyclopedia Of Industrial Chemistry, 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a05_185
- ↑ Jump up to: 6.0 6.1 6.2 Greco, C., (1978), Production of perchloromethyl mercaptan, Stauffer ChemicalCompany, Westport, Conn.
- ↑ Catalog of Chemical Suppliers, Custom Synthesis Companies and Equipment Manufactures.
External links
 |


