Biology:PETase
| PETase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
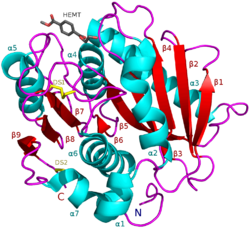 I. sakaiensis PETase (A0A0K8P6T7) in complex with HEMT, a PET analogue (PDB: 5XH3). | |||||||||
| Identifiers | |||||||||
| EC number | 3.1.1.101 | ||||||||
| Alt. names | PET hydrolase, poly(ethylene terephthalate) hydrolase | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
PETases are an esterase class of enzymes that catalyze the breakdown (via hydrolysis) of polyethylene terephthalate (PET) plastic to monomeric mono-2-hydroxyethyl terephthalate (MHET). The idealized chemical reaction is:
- (ethylene terephthalate)n + H2O → (ethylene terephthalate)n-1 + MHET,
where n is the number of monomers in the polymer chain, though a trace amount of the PET breaks down instead to bis(2-hydroxyethyl) terephthalate (BHET).[1] PETases can also break down PEF-plastic (polyethylene-2,5-furandicarboxylate), which is a bioderived PET replacement, into the analogous MHEF. PETases can't catalyze the hydrolysis of aliphatic polyesters like polybutylene succinate or polylactic acid.[2]
Whereas the degradation of PET by natural (non-enzymatic) means will take hundreds of years, PETases can degrade it in a matter of days.[3]
History
The first PETase was discovered in 2016 from Ideonella sakaiensis strain 201-F6 bacteria found from sludge samples collected close to a Japanese PET bottle recycling site.[1][4] There were other types of hydrolases previously known to degrade PET,[2] including lipases, esterases, and cutinases.[5] For comparison, enzymes that degrade polyester have been known to exist at least as far back as 1975 (in the case of α-chymotrypsin)[6] and 1977 (lipase).[7]
PET plastic came into widespread use in the 1970s and it has been suggested that PETases in bacteria evolved only recently.[2] PETase may have had past enzymatic activity associated with degradation of a waxy coating on plants.[8]
Structure
As of April 2019, there were 17 known three-dimensional crystal structures of PETases: 6QGC, 6ILX, 6ILW, 5YFE, 6EQD, 6EQE, 6EQF, 6EQG, 6EQH, 6ANE, 5XJH, 5YNS, 5XFY, 5XFZ, 5XG0, 5XH2 and 5XH3.
PETase exhibits shared qualities with both lipases and cutinases in that it possesses an α/β-hydrolase fold; although, the active-site cleft observed in PETase is more open than in cutinases.[2] The Ideonella sakaiensis PETase is similar to dienelactone hydrolase, according to Pfam. According to ESTHER, it falls into the Polyesterase-lipase-cutinase family.
There are approximately 69 PETase-like enzymes comprising a variety of diverse organisms, and there are two classifications of these enzymes including type I and type II. It is suggested that 57 enzymes fall into the type I category whereas the rest fall into the type II group, including the PETase enzyme found in the Ideonella sakaiensis. Within all 69 PETase-like enzymes, there exists the same three residues within the active site, suggesting that the catalytic mechanism is the same in all forms of PETase-like enzymes.[9]
Surface of the PETase double mutant (R103G and S131A) with HEMT (1-(2-hydroxyethyl) 4-methyl terephthalate) bound to its active site. HEMT is an analogue of MHET, and has an additional methanol esterified to it. PDBID: 5XH3.
Ribbon diagram of PETase with three residues Ser160, Asp206, and His237. The catalytic triad is represented by cyan-colored sticks. The active site is shown in orange to represent stimulation by a 2-HE(MHET)4 molecule.[9]
Mutations
The discovery of PETase from I. sakaiensis provides a potential solution to the world’s amassing plastic; however, naturally occurring enzymes are limited in their degradation abilities due to instability, low activity, and expression levels, which ultimately drive the need for improvement if they are to be used for large-scale industrial applications.[10] The majority of strategies implement site-directed mutagenesis to create an improved version, known as a variant or mutant, of the enzyme. One variant increased the activity of PETase by 22.4% by replacing the arginine with alanine in the amino acid chain at the 280th position.[11] Similarly, a double mutant was created to constrict the active site and became 4.13% more active than the wildtype.[12] Comparatively, two other double mutants created extra hydrogen bonds that improved the stability of PETase. Other successful approaches to improving PETase stability include adding Ca2+ or Mg2+, disulfide bonds and salt bridges as well as glycosylation.[10] For thermal stability, another double mutant displayed an increase in comparison to the wild type.[11] Moreover, the β1-β2 connecting loop of the enzyme may also be a future target for improved thermal stability due to its flexibility and distance from the active site.[13]
Biological pathway
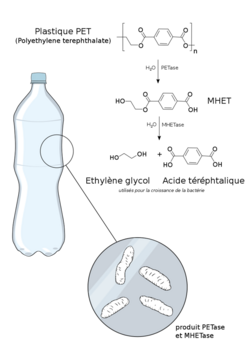
In I. sakaiensis, the resultant MHET is further broken down by the action of MHETase enzyme to terephthalic acid and ethylene glycol.[1] Laboratory experiments showed that chimeric proteins that artificially link a MHETase and a PETase outperform similar mixtures of free enzymes.[15]
See also
- Galleria mellonella, a caterpillar that can digest polyethylene.
- Aspergillus tubingensis, a fungus that can digest polyurethane.
- Pestalotiopsis microspora, an endophytic fungus species able to break down polyurethane.
- cutinase, an esterase enzyme of similar geometric shape
References
- ↑ 1.0 1.1 1.2 "A bacterium that degrades and assimilates poly(ethylene terephthalate)". Science 351 (6278): 1196–9. March 2016. doi:10.1126/science.aad6359. PMID 26965627. Bibcode: 2016Sci...351.1196Y.
- "Discovery of a Bacterium that Degrades and Assimilates Poly(ethylene terephthalate) could Serve as a Degradation and/or Fermentation Platform for Biological Recycling of PET Waste Products" (PDF). Kyoto Institute of Technology (Press release). 2016-03-30.
- ↑ 2.0 2.1 2.2 2.3 "Characterization and engineering of a plastic-degrading aromatic polyesterase". Proceedings of the National Academy of Sciences of the United States of America 115 (19): E4350–E4357. May 2018. doi:10.1073/pnas.1718804115. PMID 29666242. Bibcode: 2018PNAS..115E4350A.
- ↑ Dockrill, Peter. "Scientists Have Accidentally Created a Mutant Enzyme That Eats Plastic Waste" (in en-gb). ScienceAlert. https://www.sciencealert.com/scientists-accidentally-engineered-mutant-enzyme-eats-through-plastic-pet-petase-pollution.
- ↑ "Ideonella sakaiensis sp. nov., isolated from a microbial consortium that degrades poly(ethylene terephthalate)". International Journal of Systematic and Evolutionary Microbiology 66 (8): 2813–8. August 2016. doi:10.1099/ijsem.0.001058. PMID 27045688.
- ↑ "Structural insight into catalytic mechanism of PET hydrolase" (in En). Nature Communications 8 (1): 2106. December 2017. doi:10.1038/s41467-017-02255-z. PMID 29235460. Bibcode: 2017NatCo...8.2106H.
- ↑ Tabushi, Iwao; Yamada, Hidenori; Matsuzaki, Hidetaka; Furukawa, Junji (August 1975). "Polyester readily hydrolyzable by chymotrypsin". Journal of Polymer Science: Polymer Letters Edition 13 (8): 447–450. doi:10.1002/pol.1975.130130801. Bibcode: 1975JPoSL..13..447T.
- ↑ "Hydrolysis of polyesters by lipases". Nature 270 (5632): 76–8. November 1977. doi:10.1038/270076a0. PMID 927523. Bibcode: 1977Natur.270...76T.
- ↑ "Lab 'Accident' Becomes Mutant Enzyme That Devours Plastic". Live Science. https://www.livescience.com/62328-plastic-eating-enzyme.html.
- ↑ 9.0 9.1 "Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation" (in En). Nature Communications 9 (1): 382. January 2018. doi:10.1038/s41467-018-02881-1. PMID 29374183. Bibcode: 2018NatCo...9..382J.
- ↑ 10.0 10.1 Qi, Xinhua; Yan, Wenlong; Cao, Zhibei; Ding, Mingzhu; Yuan, Yingjin (2021-12-26). "Current Advances in the Biodegradation and Bioconversion of Polyethylene Terephthalate" (in en). Microorganisms 10 (1): 39. doi:10.3390/microorganisms10010039. ISSN 2076-2607. PMID 35056486.
- ↑ 11.0 11.1 Urbanek, Aneta K.; Kosiorowska, Katarzyna E.; Mirończuk, Aleksandra M. (2021-11-30). "Current Knowledge on Polyethylene Terephthalate Degradation by Genetically Modified Microorganisms". Frontiers in Bioengineering and Biotechnology 9. doi:10.3389/fbioe.2021.771133. ISSN 2296-4185. PMID 34917598.
- ↑ Austin, Harry P.; Allen, Mark D.; Donohoe, Bryon S.; Rorrer, Nicholas A.; Kearns, Fiona L.; Silveira, Rodrigo L.; Pollard, Benjamin C.; Dominick, Graham et al. (2018-05-08). "Characterization and engineering of a plastic-degrading aromatic polyesterase" (in en). Proceedings of the National Academy of Sciences 115 (19): E4350–E4357. doi:10.1073/pnas.1718804115. ISSN 0027-8424. PMID 29666242. Bibcode: 2018PNAS..115E4350A.
- ↑ da Costa, Clauber Henrique Souza; dos Santos, Alberto M.; Alves, Cláudio Nahum; Martí, Sérgio; Moliner, Vicent; Santana, Kauê; Lameira, Jerônimo (October 2021). "Assessment of the PETase conformational changes induced by poly(ethylene terephthalate) binding" (in en). Proteins: Structure, Function, and Bioinformatics 89 (10): 1340–1352. doi:10.1002/prot.26155. ISSN 0887-3585. PMID 34075621. https://onlinelibrary.wiley.com/doi/10.1002/prot.26155.
- ↑ Allison Chan (2016). "The Future of Bacteria Cleaning Our Plastic Waste". https://cloudfront.escholarship.org/dist/prd/content/qt7xb0c7hr/qt7xb0c7hr.pdf.
- ↑ "Characterization and engineering of a two-enzyme system for plastics depolymerization.". Proc Natl Acad Sci U S A 117 (41): 25476–25485. Oct 2020. doi:10.1073/pnas.2006753117. PMID 32989159. Bibcode: 2020PNAS..11725476K.
 |



