Biology:Listeria monocytogenes
| Listeria monocytogenes | |
|---|---|
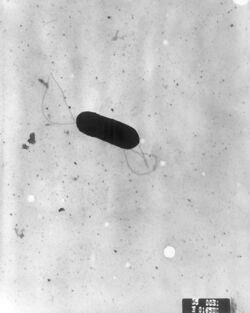
| |
| Scanning electron micrograph of Listeria monocytogenes. | |
| Scientific classification Error creating thumbnail: Unable to save thumbnail to destination
| |
| Domain: | Bacteria |
| Phylum: | Bacillota |
| Class: | Bacilli |
| Order: | Bacillales |
| Family: | Listeriaceae |
| Genus: | Listeria |
| Species: | L. monocytogenes
|
| Binomial name | |
| Listeria monocytogenes (E. Murray et al. 1926) Pirie 1940
| |
Listeria monocytogenes is the species of pathogenic bacteria that causes the infection listeriosis. It is a facultative anaerobic bacterium, capable of surviving in the presence or absence of oxygen. It can grow and reproduce inside the host's cells and is one of the most virulent foodborne pathogens: 20 to 30% of foodborne listeriosis infections in high-risk individuals may be fatal.[1][2][3] In the European Union, listeriosis follows an upward trend that began in 2008, causing 2,161 confirmed cases and 210 reported deaths in 2014, 16% more than in 2013. Listeriosis mortality rates are also higher in the EU than for other foodborne pathogens.[4][5] Responsible for an estimated 1,600 illnesses and 260 deaths in the United States annually, listeriosis ranks third in total number of deaths among foodborne bacterial pathogens, with fatality rates exceeding even Salmonella spp. and Clostridium botulinum.
Listeria monocytogenes is a Gram-positive bacterium, in the phylum Bacillota, named after Joseph Lister. Its ability to grow at temperatures as low as 0 °C permits multiplication at typical refrigeration temperatures, greatly increasing its ability to evade control in human foodstuffs. Motile via flagella at 30 °C and below, but usually not at 37 °C,[6] L. monocytogenes can instead move within eukaryotic cells by explosive polymerization of actin filaments (known as comet tails or actin rockets).[3] Once Listeria monocytogenes enters the host cytoplasm, multiple changes in bacterial metabolism and gene expression help to complete the metamorphosis of its from soil dweller to intracellular pathogen.[7]
Studies suggest up to 10% of human gastrointestinal tracts may be colonized by L. monocytogenes.[1] Nevertheless, clinical diseases due to L. monocytogenes are more frequently recognized by veterinarians, especially as meningoencephalitis in ruminants. See: listeriosis in animals.
Due to its frequent pathogenicity, causing meningitis in newborns (acquired transvaginally), pregnant mothers are often advised not to eat soft cheeses such as Brie, Camembert, feta, and queso blanco fresco, which may be contaminated with and permit growth of L. monocytogenes.[8] It is the third-most common cause of meningitis in newborns. Listeria monocytogenes can infect the brain, spinal-cord membranes and/or the bloodstream of the host[9] through the ingestion of contaminated food such as unpasteurized dairy or raw foods.[10]
Classification
L. monocytogenes is a Gram-positive, non-spore-forming, motile, facultatively anaerobic, rod-shaped bacterium. It is catalase-positive and oxidase-negative, and expresses a beta hemolysin, which causes destruction of red blood cells. This bacterium exhibits characteristic tumbling motility when viewed with light microscopy.[11] Although L. monocytogenes is actively motile by means of peritrichous flagella at room temperature (20−25 °C), the organism does not synthesize flagella at body temperatures (37 °C).[12]
The genus Listeria belongs to the class Bacilli and the order Bacillales, which also includes Bacillus and Staphylococcus. Listeria currently contains 27 species: Listeria aquatica, Listeria booriae, Listeria cornellensis, Listeria cossartiae, Listeria costaricensis, Listeria farberi, Listeria fleischmannii, Listeria floridensis, Listeria goaensis, Listeria grandensis, Listeria grayi, Listeria immobilis, Listeria innocua, Listeria ivanovii, Listeria marthii, Listeria monocytogenes, Listeria murrayi, Listeria newyorkensis, Listeria portnoyi, Listeria riparia, Listeria rocourtiae, Listeria rustica, Listeria seeligeri, Listeria thailandensis, Listeria valentina, Listeria weihenstephanensis, Listeria welshimeri. L. denitrificans, previously thought to be part of the genus Listeria, was reclassified into the new genus Jonesia.[13] Both L. ivanovii and L. monocytogenes are pathogenic in mice, but only L. monocytogenes is consistently associated with human illness.[14] The 13 serotypes of L. monocytogenes can cause disease, but more than 90% of human isolates belong to only three serotypes: 1/2a, 1/2b, and 4b. L. monocytogenes serotype 4b strains are responsible for 33 to 35% of sporadic human cases worldwide and for all major foodborne outbreaks in Europe and North America since the 1980s.[15]
History
L. monocytogenes was first described by E.G.D. Murray (Everitt George Dunne Murray) in 1924 based on six cases of sudden death in young rabbits, and published a description with his colleagues in 1926 .[16] Murray referred to the organism as Bacterium monocytogenes before Harvey Pirie changed the genus name to Listeria in 1940.[17] Although clinical descriptions of L. monocytogenes infection in both animals and humans were published in the 1920s, it was not recognized as a significant cause of neonatal infection, sepsis, and meningitis until 1952 in East Germany.[18] Listeriosis in adults was later associated with patients living with compromised immune systems, such as individuals taking immunosuppressant drugs and corticosteroids for malignancies or organ transplants, and those with HIV infection.[19]
L. monocytogenes was not identified as a cause of foodborne illness until 1981, however. An outbreak of listeriosis in Halifax, Nova Scotia, involving 41 cases and 18 deaths, mostly in pregnant women and neonates, was epidemiologically linked to the consumption of coleslaw containing cabbage that had been contaminated with L. monocytogenes-contaminated sheep manure.[20] Since then, a number of cases of foodborne listeriosis have been reported, and L. monocytogenes is now widely recognized as an important hazard in the food industry.[21]
Pathogenesis

Invasive infection by L. monocytogenes causes the disease listeriosis. When the infection is not invasive, any illness as a consequence of infection is termed febrile gastroenteritis. The manifestations of listeriosis include sepsis,[22] meningitis (or meningoencephalitis),[22] encephalitis,[23] corneal ulcer,[24] pneumonia,[25] myocarditis,[26] and intrauterine or cervical infections in pregnant women, which may result in spontaneous abortion (second to third trimester) or stillbirth. Surviving neonates of fetomaternal listeriosis may suffer granulomatosis infantiseptica — pyogenic granulomas distributed over the whole body — and may suffer from physical retardation. Influenza-like symptoms, including persistent fever, usually precede the onset of the aforementioned disorders. Gastrointestinal symptoms, such as nausea, vomiting, and diarrhea, may precede more serious forms of listeriosis or may be the only symptoms expressed. Gastrointestinal symptoms were epidemiologically associated with use of antacids or cimetidine. The onset time to serious forms of listeriosis is unknown, but may range from a few days to 3 weeks. The onset time to gastrointestinal symptoms is unknown, but probably exceeds 12 hours. An early study suggested that L. monocytogenes is unique among Gram-positive bacteria in that it might possess lipopolysaccharide,[27] which serves as an endotoxin. Later, it was found to not be a true endotoxin. Listeria cell walls consistently contain lipoteichoic acids, in which a glycolipid moiety, such as a galactosyl-glucosyl-diglyceride, is covalently linked to the terminal phosphomonoester of the teichoic acid. This lipid region anchors the polymer chain to the cytoplasmic membrane. These lipoteichoic acids resemble the lipopolysaccharides of Gram-negative bacteria in both structure and function, being the only amphipathic polymers at the cell surface.[28][29]
L. monocytogenes has D-galactose residues on its surface that can attach to D-galactose receptors on the host cell walls. These host cells are generally M cells and Peyer's patches of the intestinal mucosa. Once attached to these cells, L. monocytogenes can translocate past the intestinal membrane and into the body.[citation needed]. Alternatively, losses of structural integrity (such as small lacerations) in the gastrointestinal epithelium could allow the microorganism to penetrate from the gastrointestinal tract to the bloodstream.
The infective dose of L. monocytogenes varies with the strain and with the susceptibility of the victim. From cases contracted through raw or supposedly pasteurized milk, one may safely assume that, in susceptible persons, fewer than 1,000 total organisms may cause disease. L. monocytogenes may invade the gastrointestinal epithelium. Once the bacterium enters the host's monocytes, macrophages, or polymorphonuclear leukocytes, it becomes bloodborne (sepsis) and can grow. Its presence intracellularly in phagocytic cells also permits access to the brain and probably transplacental migration to the fetus in pregnant women. This process is known as the "Trojan Horse mechanism". The pathogenesis of L. monocytogenes centers on its ability to survive and multiply in phagocytic host cells. It seems that Listeria originally evolved to invade membranes of the intestines, as an intracellular infection, and developed a chemical mechanism to do so. This involves a bacterial protein internalin (InlA/InlB), which attaches to a protein on the intestinal cell membrane "cadherin" and allows the bacteria to invade the cells through a zipper mechanism. These adhesion molecules are also to be found in two other unusually tough barriers in humans — the blood-brain barrier and the fetal–placental barrier, and this may explain the apparent affinity that L. monocytogenes has for causing meningitis and affecting babies in utero. Once inside the cell, L. monocytogenes rapidly acidifies the lumen of the vacuole formed around it during cell entry to activate listeriolysin O, a cholesterol-dependent cytolysin capable of disrupting the vacuolar membrane. This frees the pathogen and gives it access to the cytosol of the cell, where it continues its pathogenesis.[30] Motility in the intracellular space is provided by actin assembly-inducing protein, which allows the bacteria to use the host cell's actin polymerization machinery to polymerize the cytoskeleton to give a "boost" to the bacterial cell so it can move in the cell. The same mechanism also allows the bacteria to travel from cell to cell.[citation needed]
Regulation of pathogenesis
L. monocytogenes can act as a saprophyte or a pathogen, depending on its environment. When this bacterium is present within a host organism, quorum sensing and other signals cause the up-regulation of several virulence genes. Depending on the location of the bacterium within the host organism, different activators up-regulate the virulence genes. SigB, an alternative sigma factor, up-regulates Vir genes in the intestines, whereas PrfA up-regulates gene expression when the bacterium is present in blood.[31][32][33][34] L. monocytogenes also senses the entry to host by examining available nutrient sources. For example L-glutamine, an abundant nitrogen source in the host, induces the expression of virulence genes in L. monocytogenes.[35] Little is known about how this bacterium switches between acting as a saprophyte and a pathogen; however, several noncoding RNAs are thought to be required to induce this change.[citation needed]
Pathogenicity of lineages
L. monocytogenes has three distinct lineages, with differing evolutionary histories and pathogenic potentials.[36] Lineage I strains contain the majority of human clinical isolates and all human epidemic clones, but are underrepresented in animal clinical isolates.[36] Lineage II strains are overrepresented in animal cases and underrepresented in human clinical cases, and are more prevalent in environmental and food samples.[37] Lineage III isolates are very rare, but significantly more common in animal than human isolates.[36]
Detection

The Anton test is used in the identification of L. monocytogenes; instillation of a culture into the conjunctival sac of a rabbit or guinea pig causes severe keratoconjunctivitis within 24 hours.[38][39]
Listeria species grow on media such as Mueller-Hinton agar. Identification is enhanced if the primary cultures are done on agar containing sheep blood, because the characteristic small zone of hemolysis can be observed around and under colonies. Isolation can be enhanced if the tissue is kept at 4 °C for some days before inoculation into bacteriologic media. The organism is a facultative anaerobe and is catalase-positive and motile. Listeria produces acid, but not gas, when fermenting a variety of carbohydrates.[40] The motility at room temperature and hemolysin production are primary findings that help differentiate Listeria from Corynebacterium.[41]
The methods for analysis of food are complex and time-consuming. The present U.S. FDA method, revised in September 1990, requires 24 and 48 hours of enrichment, followed by a variety of other tests. Total time to identification takes five to seven days, but the announcement of specific non-radiolabelled DNA probes should soon allow a simpler and faster confirmation of suspect isolates.[42]
Recombinant DNA technology may even permit two- to three-day positive analysis in the future. Currently, the FDA is collaborating in adapting its methodology to quantitate very low numbers of the organisms in foods.[citation needed]
Treatment
When listeric meningitis occurs, the overall mortality may reach 70%, from sepsis 50%, and from perinatal/neonatal infections greater than 80%. In infections during pregnancy, the mother usually survives. Reports of successful treatment with parenteral penicillin or ampicillin exist.[43] Trimethoprim-sulfamethoxazole has been shown effective in patients allergic to penicillin.[43]
A bacteriophage, Listeria phage P100, has been proposed as food additive to control L. monocytogenes.[44] Bacteriophage treatments have been developed by several companies. EBI Food Safety and Intralytix both have products suitable for treatment of the bacterium. The U.S. Food and Drug Administration (FDA) approved a cocktail of six bacteriophages from Intralytix, and a one-type phage product from EBI Food Safety designed to kill L. monocytogenes. Uses would potentially include spraying it on fruits and ready-to-eat meat such as sliced ham and turkey.[45]
Use as a transfection vector
Because L. monocytogenes is an intracellular bacterium, some studies have used this bacterium as a vector to deliver genes in vitro. Current transfection efficiency remains poor. One example of the successful use of L. monocytogenes in in vitro transfer technologies is in the delivery of gene therapies for cystic fibrosis cases.[46]
Cancer treatment
Listeria monocytogenes is being investigated as a cancer immunotherapy for several types of cancer.[47][48]
A live attenuated Listeria monocytogenes cancer vaccine, ADXS11-001, is under development as a possible treatment for cervical carcinoma.[49]
Epidemiology
Researchers have found Listeria monocytogenes in at least 37 mammalian species, both domesticated and feral, as well as in at least 17 species of birds and possibly in some species of fish and shellfish. Laboratories can isolate Listeria monocytogenes from soil, silage, and other environmental sources. Listeria monocytogenes is quite hardy and resists the deleterious effects of freezing, drying, and heat remarkably well for a bacterium that does not form spores. Most Listeria monocytogenes strains are pathogenic to some degree.[citation needed]
Routes of infection
Listeria monocytogenes has been associated with such foods as raw milk, pasteurized fluid milk,[50] cheeses (particularly soft-ripened varieties), hard-boiled eggs,[51] ice cream, raw vegetables, fermented raw-meat sausages, raw and cooked poultry, raw meats (of all types), and raw and smoked fish. Most bacteria can survive near freezing temperatures, but cannot absorb nutrients, grow or replicate; however, L. monocytogenes has the ability to grow at temperatures as low as 0 °C which permits exponential multiplication in refrigerated foods. At refrigeration temperature, such as 4 °C, the amount of ferric iron can affect the growth of L. monocytogenes.[52]
Infectious cycle
The primary site of infection is the intestinal epithelium, where the bacteria invade nonphagocytic cells via the "zipper" mechanism. Uptake is stimulated by the binding of listerial internalins (Inl) to E-cadherin, a host cell adhesion factor, or Met (c-Met), hepatocyte growth factor. This binding activates certain Rho-GTPases, which subsequently bind and stabilize Wiskott–Aldrich syndrome protein (WASp). WASp can then bind the Arp2/3 complex and serve as an actin nucleation point. Subsequent actin polymerization creates a "phagocytic cup", an actin-based structure normally formed around foreign materials by phagocytes prior to endocytosis. The net effect of internalin binding is to exploit the junction-forming apparatus of the host into internalizing the bacterium. L. monocytogenes can also invade phagocytic cells (e.g., macrophages), but requires only internalins for invasion of nonphagocytic cells.[citation needed]
Following internalization, the bacterium must escape from the vacuole/phagosome before fusion with a lysosome can occur. Three main virulence factors that allow the bacterium to escape are listeriolysin O (LLO encoded by hly) phospholipase A (encoded by plcA) and phospholipase B (plcB).[53][54] Secretion of LLO and PlcA disrupts the vacuolar membrane and allows the bacterium to escape into the cytoplasm, where it may proliferate.[citation needed]
Once in the cytoplasm, L. monocytogenes exploits host actin for the second time. ActA proteins associated with the old bacterial cell pole (being a bacillus, L. monocytogenes septates in the middle of the cell, thus has one new pole and one old pole) are capable of binding the Arp2/3 complex, thereby inducing actin nucleation at a specific area of the bacterial cell surface. Actin polymerization then propels the bacterium unidirectionally into the host cell membrane. The protrusion formed may then be internalized by a neighboring cell, forming a double-membrane vacuole from which the bacterium must escape using LLO and PlcB. This mode of direct cell-to-cell spread involves a cellular mechanism known as paracytophagy.[55]
The ability of L. monocytogenes to successfully infect depends on its resistance to the high concentrations of bile encountered throughout the gastrointestinal tract.[56] This resistance is due, in part, to the nucleotide excision repair protein UvrA that is necessary for repair of DNA damages caused by bile salts.[57]
References
- ↑ 1.0 1.1 "Listeria--review of epidemiology and pathogenesis". Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi 40 (1): 4–13. February 2007. PMID 17332901. http://www.sochinf.cl/documentos/infectologia/listeria.pdf. Retrieved 2010-09-05.
- ↑ "Microbe Profile: Listeria monocytogenes: a paradigm among intracellular bacterial pathogens". Microbiology 165 (7): 719–721. 2019. doi:10.1099/mic.0.000800. PMID 31124782.
- ↑ 3.0 3.1 Pizarro-Cerdá, Javier; Cossart, Pascale (2019-07-01). "Microbe Profile: Listeria monocytogenes: a paradigm among intracellular bacterial pathogens" (in en). Microbiology 165 (7): 719–721. doi:10.1099/mic.0.000800. ISSN 1350-0872. PMID 31124782.
- ↑ "Campylobacter and Listeria infections still rising in the EU – say EFSA and ECDC - European Food Safety Authority". 2015-12-17. http://www.efsa.europa.eu/en/press/news/151217.
- ↑ "The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2014". EFSA Journal 13 (12). 2015. doi:10.2903/j.efsa.2015.4329.
- ↑ "Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence". Proceedings of the National Academy of Sciences of the United States of America 101 (33): 12318–23. August 2004. doi:10.1073/pnas.0404924101. PMID 15302931. Bibcode: 2004PNAS..10112318G.
- ↑ Freitag, Nancy E. "From hot dogs to host cells: how the bacterial pathogen Listeria monocytogenes regulates virulence gene expression". https://doi.org/10.2217/17460913.1.1.89.
- ↑ "Growth and survival of Listeria monocytogenes in market cheeses stored at 4 to 30 degrees C". J. Food Prot. 54 (9): 662–668. 1991. doi:10.4315/0362-028X-54.9.662. PMID 31051570.
- ↑ "Listeriosis (Listeria infection)". https://www.health.ny.gov/diseases/communicable/listeriosis/fact_sheet.htm.
- ↑ "CDC - Sources - Listeriosis". https://www.cdc.gov/listeria/sources.html.
- ↑ "Listeria monocytogenes, a food-borne pathogen". Microbiological Reviews 55 (3): 476–511. September 1991. doi:10.1128/mr.55.3.476-511.1991. PMID 1943998.
- ↑ "Listeria monocytogenes". Todar's Online Textbook of Bacteriology. 2008. http://textbookofbacteriology.net/Listeria.html. Retrieved 28 January 2009.
- ↑ "Phylogenetic analysis of the genus Listeria based on reverse transcriptase sequencing of 16S rRNA". International Journal of Systematic Bacteriology 41 (2): 240–6. April 1991. doi:10.1099/00207713-41-2-240. PMID 1713054.
- ↑ "Chapter 15: Listeria monocytogenes". Compendium of Fish and Fishery Product Processes, Hazards, and Controls. Seafood HACCP Alliance. 2007. http://seafood.ucdavis.edu/haccp/compendium/chapt15.htm. Retrieved 28 January 2009.
- ↑ "Intraspecific phylogeny and lineage group identification based on the prfA virulence gene cluster of Listeria monocytogenes". Journal of Bacteriology 186 (15): 4994–5002. August 2004. doi:10.1128/JB.186.15.4994-5002.2004. PMID 15262937.
- ↑ "A disease of rabbits characterized by a large mononuclear leucocytosis, caused by a hitherto undescribed bacillus Bacterium monocytogenes (n. sp.)". J. Pathol. Bacteriol. 29 (4): 407–439. 1926. doi:10.1002/path.1700290409.
- ↑ "Listeria: change of name for a genus of bacteria". Nature 145 (3668): 264. 1940. doi:10.1038/145264a0. Bibcode: 1940Natur.145..264P.
- ↑ "Zur Granulomatosis infantiseptica.". Zentr. Bakteriol. I. Orig. 158 (3–5): 329–331. 1952. PMID 14959337.
- ↑ "Foodborne listeriosis". Clinical Infectious Diseases 31 (3): 770–5. September 2000. doi:10.1086/314008. PMID 11017828.
- ↑ "Epidemic listeriosis--evidence for transmission by food". The New England Journal of Medicine 308 (4): 203–6. January 1983. doi:10.1056/NEJM198301273080407. PMID 6401354.
- ↑ Listeria, Listeriosis, and Food. Safety (2nd ed.). New York: Marcel Dekker. 1999.
- ↑ 22.0 22.1 "Listeria monocytogenes and listeric infections". Bacteriological Reviews 30 (2): 309–82. June 1966. doi:10.1128/br.30.2.309-382.1966. PMID 4956900.
- ↑ "Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review". Clinical Infectious Diseases 16 (5): 689–702. May 1993. doi:10.1093/clind/16.5.689. PMID 8507761.
- ↑ "Corneal ulcer due to Listeria monocytogenes". Cornea 6 (2): 144–6. 1987. doi:10.1097/00003226-198706020-00008. PMID 3608514.
- ↑ "Listeria pneumonia. A case report". South African Medical Journal = Suid-Afrikaanse Tydskrif vir Geneeskunde 75 (4): 188–9. February 1989. PMID 2919343.
- ↑ Paras, Molly L.; Khurshid, Shaan; Foldyna, Borek; Huang, Alex L.; Hohmann, Elizabeth L.; Cooper, Leslie T.; Christensen, Bianca B. (2022-04-27). "Case 13-2022: A 56-Year-Old Man with Myalgias, Fever, and Bradycardia" (in en). New England Journal of Medicine 386 (17): 1647–1657. doi:10.1056/NEJMcpc2201233. PMID 35476654. https://www.nejm.org/doi/10.1056/NEJMcpc2201233.
- ↑ "Isolation, characterization, and biological properties of an endotoxin-like material from the gram-positive organism Listeria monocytogenes". Infection and Immunity 23 (3): 845–57. March 1979. doi:10.1128/iai.23.3.845-857.1979. PMID 110684.
- ↑ "Biochemistry of the cell surface of Listeria strains: a locating general view". Infection 16 Suppl 2 (S2): S92-7. 1988. doi:10.1007/BF01639729. PMID 3417357.
- ↑ "Listeria monocytogenes, a food-borne pathogen". Microbiological Reviews 55 (3): 476–511. September 1991. doi:10.1128/mr.55.3.476-511.1991. PMID 1943998.
- ↑ "Listeriolysin O: a genuine cytolysin optimized for an intracellular parasite". The Journal of Cell Biology 156 (6): 943–6. March 2002. doi:10.1083/jcb.200202121. PMID 11901162.
- ↑ "Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is autoregulated". Molecular Microbiology 5 (9): 2273–83. September 1991. doi:10.1111/j.1365-2958.1991.tb02158.x. PMID 1662763.
- ↑ "Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of listeria monocytogenes". Proceedings of the National Academy of Sciences of the United States of America 87 (21): 8336–40. November 1990. doi:10.1073/pnas.87.21.8336. PMID 2122460. Bibcode: 1990PNAS...87.8336L.
- ↑ "Sigma B contributes to Listeria monocytogenes gastrointestinal infection but not to systemic spread in the guinea pig infection model". Infection and Immunity 74 (2): 876–86. February 2006. doi:10.1128/IAI.74.2.876-886.2006. PMID 16428730.
- ↑ "VirR, a response regulator critical for Listeria monocytogenes virulence". Molecular Microbiology 57 (5): 1367–80. September 2005. doi:10.1111/j.1365-2958.2005.04776.x. PMID 16102006.
- ↑ "L-glutamine Induces Expression of Listeria monocytogenes Virulence Genes". PLOS Pathogens 13 (1): e1006161. January 2017. doi:10.1371/journal.ppat.1006161. PMID 28114430.
- ↑ 36.0 36.1 36.2 "Comparative genetic characterization of Listeria monocytogenes isolates from human and animal listeriosis cases". Microbiology 147 (Pt 5): 1095–104. May 2001. doi:10.1099/00221287-147-5-1095. PMID 11320113.
- ↑ "Listeria monocytogenes isolates from foods and humans form distinct but overlapping populations". Applied and Environmental Microbiology 70 (10): 5833–41. October 2004. doi:10.1128/AEM.70.10.5833-5841.2004. PMID 15466521. Bibcode: 2004ApEnM..70.5833G.
- ↑ "Anton test - definition of Anton test in the Medical dictionary - by the Free Online Medical Dictionary, Thesaurus and Encyclopedia". Medical-dictionary.thefreedictionary.com. http://medical-dictionary.thefreedictionary.com/Anton+test.
- ↑ "Anton's eye test". Whonamedit. http://www.whonamedit.com/synd.cfm/197.html.
- ↑ "Chapter 13. Non-Spore-Forming Gram-Positive Bacilli: Corynebacterium, Propionibacterium, Listeria, Erysipelothrix, Actinomycetes, & Related Pathogens". Jawetz, Melnick, & Adelberg's Medical Microbiology (24th ed.). The McGraw-Hill Companies.
- ↑ "Clinical microbiology of coryneform bacteria". Clinical Microbiology Reviews 10 (1): 125–59. January 1997. doi:10.1128/CMR.10.1.125. PMID 8993861.
- ↑ "103051F-EN-RevC". http://www.hologic.com/sites/default/files/package%20inserts/103051F-EN-RevC.pdf.
- ↑ 43.0 43.1 "Treatment of listeriosis". The Annals of Pharmacotherapy 34 (5): 656–61. May 2000. doi:10.1345/aph.19315. PMID 10852095.
- ↑ "Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application". Regulatory Toxicology and Pharmacology 43 (3): 301–12. December 2005. doi:10.1016/j.yrtph.2005.08.005. PMID 16188359.
- ↑ U.S. FDA/CFSAN: Agency Response Letter, GRAS Notice No. 000198
- ↑ "Listeria monocytogenes mediated CFTR transgene transfer to mammalian cells". The Journal of Gene Medicine 4 (6): 655–67. 2002. doi:10.1002/jgm.313. PMID 12439857.
- ↑ "Nonviral oncogenic antigens and the inflammatory signals driving early cancer development as targets for cancer immunoprevention". Clinical Cancer Research 21 (7): 1549–57. April 2015. doi:10.1158/1078-0432.CCR-14-1186. PMID 25623216.
- ↑ "Cancer immunotherapy using recombinant Listeria monocytogenes: transition from bench to clinic". Human Vaccines 7 (5): 497–505. May 2011. doi:10.4161/hv.7.5.15132. PMID 21422819.
- ↑ Lowry, Fran (2008-05-15). "Live Listeria Vaccine Proves Safe Against End-Stage Cervical Ca in Human Trial". Ob. Gyn. News 43 (10): 2.
- ↑ "Pasteurized milk as a vehicle of infection in an outbreak of listeriosis". The New England Journal of Medicine 312 (7): 404–7. February 1985. doi:10.1056/NEJM198502143120704. PMID 3918263.
- ↑ "Outbreak of Listeria Infections Linked to Hard-boiled Eggs". 4 March 2020. https://www.cdc.gov/listeria/outbreaks/eggs-12-19/index.html.
- ↑ "Influence of interactions between temperature, ferric ammonium citrate and glycine betaine on the growth of Listeria monocytogenes in a defined medium". Letters in Applied Microbiology 35 (6): 538–42. 2002. doi:10.1046/j.1472-765x.2002.01237.x. PMID 12460440.
- ↑ "Evolutionary history of the genus Listeria and its virulence genes". Systematic and Applied Microbiology 28 (1): 1–18. January 2005. doi:10.1016/j.syapm.2004.09.005. PMID 15709360.
- ↑ "Genome diversification in phylogenetic lineages I and II of Listeria monocytogenes: identification of segments unique to lineage II populations". Journal of Bacteriology 185 (18): 5573–84. September 2003. doi:10.1128/JB.185.18.5573-5584.2003. PMID 12949110.
- ↑ "Listeria monocytogenes exploits normal host cell processes to spread from cell to cell". The Journal of Cell Biology 146 (6): 1333–50. September 1999. doi:10.1083/jcb.146.6.1333. PMID 10491395.
- ↑ Merritt ME, Donaldson JR. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J Med Microbiol. 2009 Dec;58(Pt 12):1533-1541. doi: 10.1099/jmm.0.014092-0. Epub 2009 Sep 17. PMID: 19762477
- ↑ Kim SH, Gorski L, Reynolds J, Orozco E, Fielding S, Park YH, Borucki MK. Role of uvrA in the growth and survival of Listeria monocytogenes under UV radiation and acid and bile stress. J Food Prot. 2006 Dec;69(12):3031-6. doi: 10.4315/0362-028x-69.12.3031. PMID: 17186676
External links
- U.S. Food and Drug Administration. Foodborne Pathogenic Microorganisms and Natural Toxins Handbook: Listeria monocytogenes
- Public Health Agency of Canada
- Type strain of Listeria monocytogenes at BacDive - the Bacterial Diversity Metadatabase
Wikidata ☰ Q292015 entry
 |

