Chemistry:Ampicillin
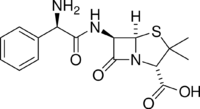 Skeletal formula of ampicillin | |
 Ball-and-stick model of the zwitterionic form of ampicillin found in the crystal structure of the trihydrate[1][2] | |
| Clinical data | |
|---|---|
| Trade names | Principen, others[3] |
| Other names | AM/AMP[4] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a685002 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth, intravenous, or intramuscular |
| Drug class | Aminopenicillins |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 62% ±17% (parenteral) < 30–55% (oral) |
| Protein binding | 15 to 25% |
| Metabolism | 12 to 50% |
| Metabolites | Penicilloic acid |
| Elimination half-life | Approx. 1 hour |
| Excretion | 75 to 85% renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C16H19N3O4S |
| Molar mass | 349.41 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ampicillin is an antibiotic belonging to the aminopenicillin class of the penicillin family. The drug is used to prevent and treat a number of bacterial infections, such as respiratory tract infections, urinary tract infections, meningitis, salmonellosis, and endocarditis.[6] It may also be used to prevent group B streptococcal infection in newborns.[6] It is used by mouth, by injection into a muscle, or intravenously.[6]
Common side effects include rash, nausea, and diarrhea.[6] It should not be used in people who are allergic to penicillin.[6] Serious side effects may include Clostridium difficile colitis or anaphylaxis.[6] While usable in those with kidney problems, the dose may need to be decreased.[6] Its use during pregnancy and breastfeeding appears to be generally safe.[6][7]
Ampicillin was discovered in 1958 and came into commercial use in 1961.[8][9] It is on the World Health Organization's List of Essential Medicines.[10] The World Health Organization classifies ampicillin as critically important for human medicine.[11] It is available as a generic medication.[6]
Medical uses
Diseases
- Bacterial meningitis; an aminoglycoside can be added to increase efficacy against gram-negative meningitis bacteria[12]
- Endocarditis by enterococcal strains (off-label use); often given with an aminoglycoside[13]
- Gastrointestinal infections caused by contaminated water or food (for example, by Salmonella)[12]
- Genito-urinary tract infections[12]
- Healthcare-associated infections that are related to infections from using urinary catheters and that are unresponsive to other medications[14]
- Otitis media (middle ear infection)
- Prophylaxis (i.e. to prevent infection) in those who previously had rheumatic heart disease or are undergoing dental procedures, vaginal hysterectomies, or C-sections.[6] It is also used in pregnant woman who are carriers of group B streptococci to prevent early-onset neonatal infections.[6]
- Respiratory infections, including bronchitis, pharyngitis[12]
- Sinusitis
- Sepsis[15]
- Whooping cough, to prevent and treat secondary infections[6]
Ampicillin used to also be used to treat gonorrhea, but there are now too many strains resistant to penicillins.[6]
Bacteria
Ampicillin is used to treat infections by many gram-positive and gram-negative bacteria. It was the first "broad spectrum" penicillin with activity against gram-positive bacteria, including Streptococcus pneumoniae, Streptococcus pyogenes, some isolates of Staphylococcus aureus (but not penicillin-resistant or methicillin-resistant strains), Trueperella, and some Enterococcus. It is one of the few antibiotics that works against multidrug resistant Enterococcus faecalis and E. faecium.[16] Activity against gram-negative bacteria includes Neisseria meningitidis, some Haemophilus influenzae, and some of the Enterobacteriaceae (though most Enterobacteriaceae and Pseudomonas are resistant).[16][17] Its spectrum of activity is enhanced by co-administration of sulbactam, a drug that inhibits beta lactamase, an enzyme produced by bacteria to inactivate ampicillin and related antibiotics.[18][19] It is sometimes used in combination with other antibiotics that have different mechanisms of action, like vancomycin, linezolid, daptomycin, and tigecycline.[20][21]
Available forms
Ampicillin can be administered by mouth, an intramuscular injection (shot) or by intravenous infusion.[6] The oral form, available as capsules or oral suspensions, is not given as an initial treatment for severe infections, but rather as a follow-up to an IM or IV injection.[6] For IV and IM injections, ampicillin is kept as a powder that must be reconstituted.[22]
IV injections must be given slowly, as rapid IV injections can lead to convulsive seizures.[6][23]
Specific populations
Ampicillin is one of the most used drugs in pregnancy,[24] and has been found to be generally harmless both by the Food and Drug Administration in the U.S. (which classified it as category B) and the Therapeutic Goods Administration in Australia (which classified it as category A).[6][25] It is the drug of choice for treating Listeria monocytogenes in pregnant women, either alone or combined with an aminoglycoside.[6] Pregnancy increases the clearance of ampicillin by up to 50%, and a higher dose is thus needed to reach therapeutic levels.[24][26]
Ampicillin crosses the placenta and remains in the amniotic fluid at 50–100% of the concentration in maternal plasma; this can lead to high concentrations of ampicillin in the newborn.[26]
While lactating mothers secrete some ampicillin into their breast milk, the amount is minimal.[6][24]
In newborns, ampicillin has a longer half-life and lower plasma protein binding.[27] The clearance by the kidneys is lower, as kidney function has not fully developed.[6]
Contraindications
Ampicillin is contraindicated in those with a hypersensitivity to penicillins, as they can cause fatal anaphylactic reactions. Hypersensitivity reactions can include frequent skin rashes and hives, exfoliative dermatitis, erythema multiforme, and a temporary decrease in both red and white blood cells.[12]
Ampicillin is not recommended in people with concurrent mononucleosis, as over 40% of patients develop a skin rash.[12]
Side effects
Ampicillin is comparatively less toxic than other antibiotics, and side effects are more likely in those who are sensitive to penicillins and those with a history of asthma or allergies.[12] In very rare cases, it causes severe side effects such as angioedema, anaphylaxis, and C. difficile infection (that can range from mild diarrhea to serious pseudomembranous colitis).[12] Some develop black "furry" tongue. Serious adverse effects also include seizures and serum sickness. The most common side effects, experienced by about 10% of users are diarrhea and rash. Less common side effects can be nausea, vomiting, itching, and blood dyscrasias. The gastrointestinal effects, such as hairy tongue, nausea, vomiting, diarrhea, and colitis, are more common with the oral form of penicillin.[12] Other conditions may develop up several weeks after treatment.[6]
Overdose
Ampicillin overdose can cause behavioral changes, confusion, blackouts, and convulsions, as well as neuromuscular hypersensitivity, electrolyte imbalance, and kidney failure.[12]
Interactions
Ampicillin reacts with probenecid and methotrexate to decrease renal excretion. Large doses of ampicillin can increase the risk of bleeding with concurrent use of warfarin and other oral anticoagulants, possibly by inhibiting platelet aggregation.[28] Ampicillin has been said to make oral contraceptives less effective,[6] but this has been disputed.[29] It can be made less effective by other antibiotic, such as chloramphenicol, erythromycin, cephalosporins, and tetracyclines.[22] For example, tetracyclines inhibit protein synthesis in bacteria, reducing the target against which ampicillin acts.[30] If given at the same time as aminoglycosides, it can bind to it and inactivate it. When administered separately, aminoglycosides and ampicillin can potentiate each other instead.[6][31]
Ampicillin causes skin rashes more often when given with allopurinol.[12]
Both the live cholera vaccine and live typhoid vaccine can be made ineffective if given with ampicillin. Ampicillin is normally used to treat cholera and typhoid fever, lowering the immunological response that the body has to mount.[32][33][34]
Pharmacology
Mechanism of action

Ampicillin is in the penicillin group of beta-lactam antibiotics and is part of the aminopenicillin family. It is roughly equivalent to amoxicillin in terms of activity.[6] Ampicillin is able to penetrate gram-positive and some gram-negative bacteria. It differs from penicillin G, or benzylpenicillin, only by the presence of an amino group. This amino group, present on both ampicillin and amoxicillin, helps these antibiotics pass through the pores of the outer membrane of gram-negative bacteria, such as E. coli, Proteus mirabilis, Salmonella enterica, and Shigella.[18][35]
Ampicillin acts as an irreversible inhibitor of the enzyme transpeptidase, which is needed by bacteria to make the cell wall.[6] It inhibits the third and final stage of bacterial cell wall synthesis in binary fission, which ultimately leads to cell lysis; therefore, ampicillin is usually bacteriolytic.[6][36]
Pharmacokinetics
Ampicillin is well-absorbed from the GI tract (though food reduces its absorption), and reaches peak concentrations in one to two hours. The bioavailability is around 62% for parenteral routes. Unlike other penicillins, which usually bind 60–90% to plasma proteins, ampicillin binds to only 15–20%.[6][27]
Ampicillin is distributed through most tissues, though it is concentrated in the liver and kidneys. It can also be found in the cerebrospinal fluid when the meninges become inflamed (such as, for example, meningitis).[27] Some ampicillin is metabolized by hydrolyzing the beta-lactam ring to penicilloic acid,[6] though most of it is excreted unchanged.[12] In the kidneys, it is filtered out mostly by tubular secretion; some also undergoes glomerular filtration, and the rest is excreted in the feces and bile.
Hetacillin and pivampicillin are ampicillin esters that have been developed to increase bioavailability.[37]
History
Ampicillin has been used extensively to treat bacterial infections since 1961.[38] Until the introduction of ampicillin by the British company Beecham, penicillin therapies had only been effective against gram-positive organisms such as staphylococci and streptococci.[36] Ampicillin (originally branded as "Penbritin") also demonstrated activity against gram-negative organisms such as H. influenzae, coliforms, and Proteus spp.[38]
Cost
Ampicillin is relatively inexpensive.[39] In the United States, it is available as a generic medication.[6]
Veterinary use
In veterinary medicine, ampicillin is used in cats, dogs, and farm animals to treat:[15]
- Anal gland infections
- Cutaneous infections, such as abscesses, cellulitis, and pustular dermatitis
- E. coli and Salmonella infections in cattle, sheep, and goats (oral form). Ampicillin use for this purpose had declined as bacterial resistance has increased.[37]
- Mastitis in sows[40]
- Mixed aerobic–anaerobic infections, such as from cat bites[37]
- Multidrug-resistant Enterococcus faecalis and E. faecium[16]
- Prophylactic use in poultry against Salmonella and sepsis from E. coli or Staphylococcus aureus[37]
- Respiratory tract infections, including tonsilitis, bovine respiratory disease, shipping fever, bronchopneumonia, and calf and bovine pneumonia
- Urinary tract infections in dogs
Horses are generally not treated with oral ampicillin, as they have low bioavailability of beta-lactams.[16]
The half-life in animals is around that same of that in humans (just over an hour). Oral absorption is less than 50% in cats and dogs, and less than 4% in horses.[17]
See also
- Amoxycillin (p-hydroxy metabolite of ampicillin)
- Azlocillin and pirbenicillin (urea and amide made from ampicillin)
- Pivampicillin (special pro-drug of ampicillin)
References
- ↑ CSD Entry: AMPCIH01. Cambridge Crystallographic Data Centre. 2006. doi:10.5517/ccb0nkj. https://dx.doi.org/10.5517/ccb0nkj. Retrieved 2022-06-28.
- ↑ "Ampicillin trihydrate from synchrotron powder diffraction data". Acta Crystallogr. E 62 (2): o797–o799. 2006. doi:10.1107/S1600536806001371. Bibcode: 2006AcCrE..62O.797B.
- ↑ "Ampicillin - international drug names". 30 November 2019. https://www.drugs.com/international/ampicillin.html.
- ↑ "Antibiotic abbreviations list". https://microbiologie-clinique.com/antibiotic-family-abbreviation.html.
- ↑ 5.0 5.1 "Ampicillin Use During Pregnancy". 2 May 2019. https://www.drugs.com/pregnancy/ampicillin.html.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 6.18 6.19 6.20 6.21 6.22 6.23 6.24 6.25 6.26 6.27 6.28 6.29 "Ampicillin". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/ampicillin.html.
- ↑ "Ampicillin use while Breastfeeding". March 2015. https://www.drugs.com/breastfeeding/ampicillin.html.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 490. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA490.
- ↑ The evolution of drug discovery : from traditional medicines to modern drugs (1 ed.). Weinheim: Wiley-VCH. 2011. p. 262. ISBN 9783527326693. https://books.google.com/books?id=iDNy0XxGqT8C&pg=PA262.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Critically important antimicrobials for human medicine (6th revision ed.). Geneva: World Health Organization. 2019. ISBN 9789241515528.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 "Ampicillin- ampicillin sodium injection, powder, for solution". 11 September 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0642ef5e-7eff-4117-a0df-4bd15d7b7d63.
- ↑ WebMD. "ampicillin (Rx)". Medscape. http://reference.medscape.com/drug/ampi-omnipen-ampicillin-342475.
- ↑ "Chapter 132: Infections in Transplant Recipients". Harrison's Principles of Internal Medicine. 1 (18th ed.). McGraw-Hill Medical. 2012. ISBN 978-0-07-174887-2.
- ↑ 15.0 15.1 "Ampicillin injection, powder, for suspension". 26 October 2017. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=af6eb56d-698f-43aa-bb3b-a0a69732a7ae.
- ↑ 16.0 16.1 16.2 16.3 Equine Pharmacology, an Issue of Veterinary Clinics of North America: Equine Practice, E-Book. Elsevier Health Sciences. 2017. pp. 59. ISBN 978-0-323-52438-4. https://books.google.com/books?id=AiV4DgAAQBAJ&pg=PT59.
- ↑ 17.0 17.1 Saunders Handbook of Veterinary Drugs: Small and Large Animal. Elsevier Health Sciences. 2015. pp. 43–47. ISBN 978-0-323-24485-5. https://books.google.com/books?id=ip8_CwAAQBAJ&pg=PA43.
- ↑ 18.0 18.1 Antibiotic Basics for Clinicians: The ABCs of Choosing the Right Antibacterial Agent. Lippincott Williams & Wilkins. 2012. pp. 25–28. ISBN 978-1-4511-1221-4. https://books.google.com/books?id=17pMk0v3_bYC&pg=PA25.
- ↑ "Sulbactam-containing beta-lactamase inhibitor combinations". Clinical Microbiology and Infection 14 (Suppl 1): 185–188. January 2008. doi:10.1111/j.1469-0691.2007.01847.x. PMID 18154545.
- ↑ "Safety and efficacy of commonly used antimicrobial agents in the treatment of enterococcal infections: a review". Expert Opinion on Drug Safety 15 (2): 153–167. 2016. doi:10.1517/14740338.2016.1127349. PMID 26629598.
- ↑ (in en) Oxford Handbook of Infectious Diseases and Microbiology. Oxford University Press. December 2016. p. 721. ISBN 9780191503108. https://books.google.com/books?id=G4KuDQAAQBAJ&pg=PA721.
- ↑ 22.0 22.1 Applied Pharmacology for Veterinary Technicians - E-Book. Elsevier Health Sciences. 2014. pp. 244. ISBN 978-0-323-29170-5. https://books.google.com/books?id=7s3sAwAAQBAJ&pg=PA243.
- ↑ WebMD. "Ampicillin Intravenous: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing". WebMD. https://www.webmd.com/drugs/2/drug-18412/ampicillin-intravenous/details.
- ↑ 24.0 24.1 24.2 The Complete Guide to Medications During Pregnancy and Breastfeeding: Everything You Need to Know to Make the Best Choices for You and Your Baby. St. Martin's Press. 2013. pp. 47–49. ISBN 978-1-250-03720-6. https://archive.org/details/completeguidetom0000wein_t9k5. "ampicillin pregnancy."
- ↑ Australian Government Department of Health Therapeutic Goods Administration (25 October 2017). "Prescribing medicines in pregnancy database". Therapeutic Goods Administration (TGA). Australian Government Department of Health. https://www.tga.gov.au/prescribing-medicines-pregnancy-database. Note: scroll down to "Search by name" and type "ampicillin" into the search bar. Requires JavaScript to be enabled.
- ↑ 26.0 26.1 Infectious Diseases in Critical Care. Springer Science & Business Media. 2010. pp. 172. ISBN 978-3-540-34406-3. https://books.google.com/books?id=wysQmU5bYAAC&pg=PA172.
- ↑ 27.0 27.1 27.2 Essential Drug Data for Rational Therapy in Veterinary Practice. AuthorHouse. 2014. pp. 26. ISBN 978-1-4918-0010-2. https://books.google.com/books?id=CtfIAgAAQBAJ&pg=PA26.
- ↑ "Drug interactions between ampicillin and anisindione". Drugs.com. https://www.drugs.com/drug-interactions/ampicillin-with-anisindione-196-0-210-0.html?professional=1.
- ↑ Drug Interactions in Infectious Diseases. Springer Science & Business Media. 2011. pp. 213–214. ISBN 978-1-61779-213-7. https://books.google.com/books?id=94x8C5pbhqoC&pg=PA213.
- ↑ "Drug interactions between ampicillin and bismuth subcitrate potassium / metronidazole / tetracycline". Drugs.com. https://www.drugs.com/drug-interactions/ampicillin-with-bismuth-subcitrate-potassium-metronidazole-tetracycline-196-0-388-0.html?professional=1.
- ↑ "Drug interactions between amikacin and ampicillin". Drugs.com. https://www.drugs.com/drug-interactions/amikacin-with-ampicillin-153-0-196-0.html?professional=1.
- ↑ "Drug interactions between ampicillin and cholera vaccine, live". Drugs.com. https://www.drugs.com/drug-interactions/ampicillin-with-cholera-vaccine-live-196-0-3772-0.html?professional=1.
- ↑ "Drug interactions between ampicillin and typhoid vaccine, live". Drugs.com. https://www.drugs.com/drug-interactions/ampicillin-with-typhoid-vaccine-live-196-0-2271-0.html?professional=1.
- ↑ "Cholera Medication". Medscape. 22 June 2017. https://emedicine.medscape.com/article/962643-medication.
- ↑ "Outer membrane permeability and antibiotic resistance". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1794 (5): 808–816. May 2009. doi:10.1016/j.bbapap.2008.11.005. PMID 19100346.
- ↑ 36.0 36.1 "Penicillins, Cephalosporins, and Other Beta-Lactam Antibiotics". Goodman and Gilman's the Pharmacological Basis of Therapeutics (Twelfth ed.). New York: McGraw Hill Professional. 2011. pp. 1477–1504. ISBN 978-0-07-176939-6.
- ↑ 37.0 37.1 37.2 37.3 Antimicrobial Therapy in Veterinary Medicine. John Wiley & Sons. 2013. pp. 167–170. ISBN 978-1-118-67507-6. https://books.google.com/books?id=ybA2AAAAQBAJ&pg=RA2-PT166.
- ↑ 38.0 38.1 "Pharmacology and chemotherapy of ampicillin--a new broad-spectrum penicillin". British Journal of Pharmacology and Chemotherapy 18 (2): 356–369. April 1962. doi:10.1111/j.1476-5381.1962.tb01416.x. PMID 13859205.
- ↑ (in en) Penn Clinical Manual of Urology E-Book. Elsevier Health Sciences. 2014. p. 122. ISBN 978-0-323-24466-4. https://books.google.com/books?id=OQTbAgAAQBAJ&pg=PA122.
- ↑ "Mastitis in Sows". Merck Veterinary Manual. http://www.merckvetmanual.com/reproductive-system/mastitis-in-large-animals/mastitis-in-sows.
External links
- "Ampicillin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/ampicillin.
- Frank Peter Doyle, John Herbert Charles Nayler, Harry Smith, "Penicillins", GB patent 902703, published 1962-08-09, assigned to Beecham Research Laboratories Ltd
- Frank Peter Doyle, John Herbert Charles Nayler, Harry Smith, "Alpha-aminobenzylpenicillins", US patent 2985648, published 1961-05-23, issued 1961-05-23
- & Glenn A Hardcastle Jr"D-(-)-alpha-aminobenzylpenicillin trihydrate" US patent 3157640, published 1964-11-17, issued 1964-11-17, assigned to Bristol Myers Co
 |
