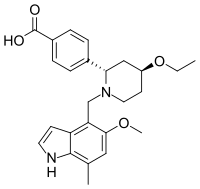
Chemistry:Iptacopan
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Fabhalta |
| Other names | LNP023 |
| AHFS/Drugs.com | Fabhalta |
| License data | |
| Routes of administration | By mouth |
| Drug class | Complement factor B inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C25H30N2O4 |
| Molar mass | 422.525 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Iptacopan , sold under the brand name Fabhalta, is a medication used for the treatment of paroxysmal nocturnal hemoglobinuria.[1] It is a complement factor B inhibitor that was developed by Novartis.[1] It is taken by mouth.[1]
Iptacopan was approved by the US Food and Drug Administration (FDA) for the treatment of adults with paroxysmal nocturnal hemoglobinuria in December 2023.[2][3] The FDA considers it to be a first-in-class medication.[4]
Medical uses
Iptacopan is indicated for the treatment of adults with paroxysmal nocturnal hemoglobinuria.[1][5]
Mechanism of action
Iptacopan binds to Factor B of the alternative complement pathway and regulates the cleavage of C3, generation of downstream effectors, and the amplification of the terminal pathway.[6]
In PNH, intravascular hemolysis (IVH) is mediated by the downstream membrane attack complex (MAC), while extravascular hemolysis (EVH) is facilitated by C3b opsonization. Iptacopan acts proximally in the alternative pathway of the complement cascade to control both C3b-mediated EVH and terminal complement mediated IVH.[7]
Side effects
The FDA label for iptacopan contains a black box warning for the risk of serious and life-threatening infections caused by encapsulated bacteria, including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B.[1]
Research
In a clinical study with twelve participants, iptacopan as a single drug led to the normalization of hemolytic markers in most patients, and no serious adverse events occurred during the 12-week study.[8][9]
Iptacopan is also investigated as a drug in other complement-mediated diseases, like age-related macular degeneration and some types of glomerulopathies.[10]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Fabhalta- iptacopan capsule". 5 December 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a76b5845-6e21-4d3b-ad07-cd8df1b60bee.
- ↑ "Novartis receives FDA approval for Fabhalta (iptacopan), offering superior hemoglobin improvement in the absence of transfusions as the first oral monotherapy for adults with PNH". Novartis (Press release). Archived from the original on 12 December 2023. Retrieved 6 December 2023.
- ↑ "Novel Drug Approvals for 2023". 6 December 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023.
- ↑ (PDF) New Drug Therapy Approvals 2023 (Report). January 2024. https://www.fda.gov/media/175253/download. Retrieved 9 January 2024.
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/218276Orig1s000ltr.pdf
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ https://www.novartis.com/us-en/sites/novartis_us/files/fabhalta.pdf
- ↑ https://www.novartis.com/us-en/sites/novartis_us/files/fabhalta.pdf
- ↑ "Iptacopan monotherapy in patients with paroxysmal nocturnal hemoglobinuria: a 2-cohort open-label proof-of-concept study". Blood Advances 6 (15): 4450–4460. August 2022. doi:10.1182/bloodadvances.2022006960. PMID 35561315.
- ↑ "Novartis Phase III APPOINT-PNH trial shows investigational oral monotherapy iptacopan improves hemoglobin to near-normal levels, leading to transfusion independence in all treatment-naïve PNH patients". Novartis (Press release). Archived from the original on 12 December 2023. Retrieved 6 September 2023.
- ↑ "Small-molecule factor B inhibitor for the treatment of complement-mediated diseases". Proceedings of the National Academy of Sciences of the United States of America 116 (16): 7926–7931. April 2019. doi:10.1073/pnas.1820892116. PMID 30926668. Bibcode: 2019PNAS..116.7926S.
External links
- Clinical trial number NCT04558918 for "Study of Efficacy and Safety of Twice Daily Oral LNP023 in Adult PNH Patients With Residual Anemia Despite Anti-C5 Antibody Treatment (APPLY-PNH)" at ClinicalTrials.gov
- Clinical trial number NCT04820530 for "Study of Efficacy and Safety of Twice Daily Oral Iptacopan (LNP023) in Adult PNH Patients Who Are Naive to Complement Inhibitor Therapy (APPOINT-PNH)" at ClinicalTrials.gov
 |

