Chemistry:Thiocarbonic acid
| |
| Names | |
|---|---|
| IUPAC name
Carbonotrithioic acid
| |
| Systematic IUPAC name
Trithiocarbonic acid | |
| Other names
Thiocarbonic acid
Sulfocarbonic acid Trisulfocarbonic acid Dithiocarbon sulfide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| MeSH | C013321 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| H 2CS 3 | |
| Molar mass | 110.21 g·mol−1 |
| Appearance | Red oily liquid, yellow solid |
| Density | 1.483 g/cm3 (liquid) |
| Melting point | −26.8 °C; −16.3 °F; 246.3 K |
| Boiling point | 58 °C; 136 °F; 331 K |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
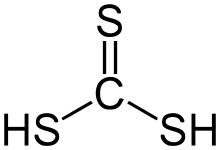
Thiocarbonic acid is an inorganic acid with the chemical formula H
2CS
3 (or S=C(SH)
2). It is an analog of carbonic acid H
2CO
3 (or O=C(OH)
2), in which all oxygen atoms are replaced with sulfur atoms. It is an unstable hydrophobic red oily liquid.[1]
It is often referred to as trithiocarbonic acid so as to differentiate it from other carbonic acids containing sulfur, such as monothiocarbonic O,O-acid S=C(OH)
2, monothiocarbonic O,S-acid O=C(OH)(SH), dithiocarbonic O,S-acid S=C(OH)(SH) and dithiocarbonic S,S-acid O=C(SH)
2 (see thiocarbonates).
Discovery and synthesis
It was first reported in brief by Zeise in 1824 and later in more detail by Berzelius in 1826,[2] in both cases it was produced by the action of carbon disulfide on a hydrosulfide salt (e.g. potassium hydrosulfide).[3]
Treatment with acids liberates the thiocarbonic acid as a red oil
- K
2CS
3 + 2 HX → H
2CS
3 + 2 KX
Both the acid and many of its salts are unstable and decompose via the release of carbon disulfide, particularly upon heating:
- H
2CS
3 → CS
2 + H
2S
An improved synthesis involves addition of barium trithiocarbonate to hydrochloric acid at 0 °C. This method provided samples with which many measurement have been made.[1]
Despite its lability, crystals of thiocarbonic acid have been examined by X-ray crystallography, which confirms the anticipated molecular structure of a trigonal–planar central carbon atom. The C-S bond lengths range from 1.69 to 1.77 Å.[4]
Reactions and derivatives
Thiocarbonic acid is acidic, with the first pKa being −2.3. The second pKa is near 7. It dissolves S
8, but does not react with it.[1]
Salts and esters of trithiocarbonic acid are called trithiocarbonates, and they are sometimes called thioxanthates.
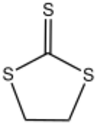
Thiocarbonic acid reacts with bifunctional reagents to give rings. 1,2-Dichloroethane gives ethylene trithiocarbonate (S=CS
2(CH
2)
2). Oxalyl chloride gives oxalyl trithiocarbonate (S=CS
2(C=O)
2).
-
Oxalyl trithiocarbonate
Applications
Thiocarbonic acid currently has no significant applications. Its esters find use in RAFT polymerization.
References
- ↑ 1.0 1.1 1.2 Gattow, Gerhard; Behrendt, Werner (1977). Carbon Sulfides and their Inorganic and Complex Chemistry. Stuttgart: Georg Thieme. p. 154-6. ISBN 3135262014.
- ↑ Berzelius, J. J. (1826). "Ueber die Schwefelsalze" (in German). Annalen der Physik 82 (4): 425–458. doi:10.1002/andp.18260820404. Bibcode: 1826AnP....82..425B. https://zenodo.org/record/1423508.
- ↑ O'Donoghue, Ida Guinevere; Kahan, Zelda (1906). "CLXXIV.—Thiocarbonic acid and some of its salts". J. Chem. Soc., Trans. 89: 1812–1818. doi:10.1039/CT9068901812. https://zenodo.org/record/2186178.
- ↑ Krebs, B.; Gattow, G. (1965). "Über Chalkogenocarbonate. XIV. Das Kohlenstoffsulfid-bis-(hydrogensulfid) SC(SH)2 und das System H2S−CS2 6. Die Kristallstruktur der Trithiokohlensure bei -100 ″C". Zeitschrift für anorganische und allgemeine Chemie 340 (5–6): 294–311. doi:10.1002/zaac.19653400508.
 |