Biology:Aurora inhibitor
Aurora kinase inhibitors are a putative drug class for treating cancer. The Aurora kinase enzymes could be potential targets for novel small-molecule enzyme inhibitors.
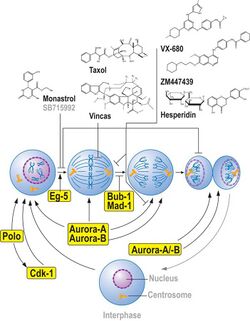
Aurora kinases regulate cell cycle transit from G2 through cytokinesis, and thus are targets in cancer therapy.[1] There are three mammalian aurora kinase genes, encoding aurora A, B and C. Intense investigation has focused on aurora A and B as they appear to play a role in oncogenesis[2] with aurora A identified as a low penetrance tumor susceptibility gene in mice and humans.[3]
Drug development
A new approach to inhibiting cancer growth that shows great promise for structure-based drug development is targeting enzymes central to cellular mitosis.[4] Aurora kinases, so named because the scattered mitotic spindles generated by mutant forms resemble the Aurora Borealis, have gained a great deal of attention as possible anticancer drug targets.[5][6] The Aurora enzymes are particularly significant because they are involved in a direct path to the nucleosome by phosphorylating histone H3.[7][8] Furthermore, Aurora kinases are known to be oncogenic and overexpressed in various forms of cancerous growth, including leukemia, colon cancer, prostate cancer[9] and breast cancer[10] tumors.[11]
So far three Aurora-kinase inhibitors have been described: ZM447439,[12] hesperadin[13][14] and VX-680. The last is in advanced stages (Phase II clinical trial) of a joint drug development by Vertex Pharmaceuticals's VX-680 (Sausville, 234, last posted on 12/18/06) and Merck & Co.,[15] although the Phase II clinical trial was suspended in November, 2007 due to QT prolongation observed in one patient in Phase I trial.
-
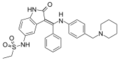
VX-680
Aurora structure

The structure and active site of Aurora-2-adenosine complex has been determined.[16] The hinge (yellow), glycine-rich loop (blue), and activation loop (red) are key features of the protein kinase fold involved in binding adenosine. The protein backbone atoms of residues Glu-211, Ala-213 in the hinge region of Aurora-2, and the sidechain of residue Trp-277, located in the activation loop, bind adenosine through specific hydrogen bonds. There are no hydrogen bonds between the 2'-OH or 3'-OH groups of the ribose moiety and Aurora-2. Residues Lys-162 and Asp-274 are essential for Aurora-2 kinase activity but do not hydrogen bond to each other as seen in crystal structures of several other protein kinases.[citation needed]
See also
References
- ↑ Andrews, Paul D.; Knatko, Elena; Moore, William J.; Swedlow, Jason R. (2003). "Mitotic mechanics: The auroras come into view". Current Opinion in Cell Biology 15 (6): 672–683. doi:10.1016/j.ceb.2003.10.013. PMID 14644191.
- ↑ Katayama, Hiroshi; Brinkley, W. R.; Sen, S. (2003). "The Aurora kinases: Role in cell transformation and tumorigenesis". Cancer and Metastasis Reviews 22 (4): 451–464. doi:10.1023/a:1023789416385. PMID 12884918.
- ↑ Ewart-Toland, Amanda; Briassouli, Paraskevi; De Koning, John P.; Mao, Jian-Hua; Yuan, Jinwei; Chan, Florence; Maccarthy-Morrogh, Lucy; Ponder, Bruce A J. et al. (2003). "Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human". Nature Genetics 34 (4): 403–412. doi:10.1038/ng1220. PMID 12881723.
- ↑ Nigg, Erich A. (2001). "Mitotic kinases as regulators of cell division and its checkpoints". Nature Reviews Molecular Cell Biology 2 (1): 21–32. doi:10.1038/35048096. PMID 11413462.
- ↑ Keen, Nicholas; Taylor, Stephen (2004). "Aurora-kinase inhibitors as anticancer agents". Nature Reviews Cancer 4 (12): 927–936. doi:10.1038/nrc1502. PMID 15573114.
- ↑ Carvajal, R. D.; Tse, A.; Schwartz, G. K. (2006). "Aurora Kinases: New Targets for Cancer Therapy". Clinical Cancer Research 12 (23): 6869–6875. doi:10.1158/1078-0432.CCR-06-1405. PMID 17145803.
- ↑ Goto, Hidemasa; Yasui, Yoshihiro; Nigg, Erich A.; Inagaki, Masaki (2002). "Aurora-B phosphorylates Histone H3 at serine28 with regard to the mitotic chromosome condensation". Genes to Cells 7 (1): 11–17. doi:10.1046/j.1356-9597.2001.00498.x. PMID 11856369.
- ↑ Monier, K.; Mouradian, S.; Sullivan, K. F. (2006). "DNA methylation promotes Aurora-B-driven phosphorylation of histone H3 in chromosomal subdomains". Journal of Cell Science 120 (Pt 1): 101–114. doi:10.1242/jcs.03326. PMID 17164288.
- ↑ Lee, Edmund Chun Yu; Frolov, Anna; Li, Rile; Ayala, Gustavo; Greenberg, Norman M. (2006). "Targeting Aurora Kinases for the Treatment of Prostate Cancer". Cancer Research 66 (10): 4996–5002. doi:10.1158/0008-5472.CAN-05-2796. PMID 16707419.
- ↑ Yang, Hua; Ou, Chien Chen; Feldman, Richard I.; Nicosia, Santo V.; Kruk, Patricia A.; Cheng, Jin Q. (2004). "Aurora-A Kinase Regulates Telomerase Activity through c-Myc in Human Ovarian and Breast Epithelial Cells". Cancer Research 64 (2): 463–467. doi:10.1158/0008-5472.can-03-2907. PMID 14744757.
- ↑ Fu, J.; Bian, M.; Jiang, Q.; Zhang, C. (2007). "Roles of Aurora Kinases in Mitosis and Tumorigenesis". Molecular Cancer Research 5 (1): 1–10. doi:10.1158/1541-7786.MCR-06-0208. PMID 17259342.
- ↑ Gadea, Bedrick B.; Ruderman, Joan V. (2005). "Aurora Kinase Inhibitor ZM447439 Blocks Chromosome-induced Spindle Assembly, the Completion of Chromosome Condensation, and the Establishment of the Spindle Integrity Checkpoint inXenopus Egg Extracts". Molecular Biology of the Cell 16 (3): 1305–1318. doi:10.1091/mbc.e04-10-0891. PMID 15616188.
- ↑ Peters, Jan-Michael; Rieder, Conly L.; Van Meel, Jacques; Heckel, Armin; Walter, Rainer; Schnapp, Gisela; Zimmer, Christine; Laterra, Sabrina et al. (2003). "The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore–microtubule attachment and in maintaining the spindle assembly checkpoint". Journal of Cell Biology 161 (2): 281–294. doi:10.1083/jcb.200208092. PMID 12707311.
- ↑ Sakita-Suto, Shiho; Kanda, Akifumi; Suzuki, Fumio; Sato, Sunao; Takata, Takashi; Tatsuka, Masaaki (2007). "Aurora-B Regulates RNA Methyltransferase NSUN2". Molecular Biology of the Cell 18 (3): 1107–1117. doi:10.1091/mbc.e06-11-1021. PMID 17215513.
- ↑ Harrington, Elizabeth A.; Bebbington, David; Moore, Jeff; Rasmussen, Richele K.; Ajose-Adeogun, Abi O.; Nakayama, Tomoko; Graham, Joanne A.; Demur, Cecile et al. (2004). "VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo". Nature Medicine 10 (3): 262–267. doi:10.1038/nm1003. PMID 14981513.
- ↑ Graham M. T. et al., Crystal Structure of Aurora-2, an Oncogenic Serine/Threonine Kinase J. Biol. Chem., (2002) 277: pp.42419-22
External links
- Aurora-A (Serine/threonine-protein kinase 6, AIK, ARK1, AURA, BTAK, STK15, STK6)
- Aurora-B (Serine/threonine-protein kinase 12, AIK2, AIM1, ARK2, STK12)
- Aurora-C (Serine/threonine-protein kinase 13, AIE2, AIK3, STK13)
- Mitotic spindle & Aurora - PMAP The Proteolysis Map-animation
 |