Chemistry:Gepotidacin
From HandWiki
(Redirected from Chemistry:C24H28N6O3)
Short description: Chemical compound
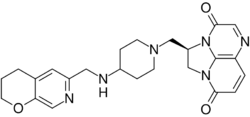 | |
| Clinical data | |
|---|---|
| Other names | GSK2140944 |
| ATC code |
|
| Identifiers | |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C24H28N6O3 |
| Molar mass | 448.527 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Gepotidacin (INN[1]) is an experimental antibiotic that acts as a topoisomerase type II inhibitor.[2] It is being studied for the treatment of uncomplicated urinary tract infection (acute cystitis)[3] and infection with Neisseria gonorrhoeae (gonorrhea), including multidrug resistant strains.[4][5]
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances". WHO Drug Information 28 (4). 2014. https://www.who.int/medicines/publications/druginformation/PL112-final.pdf.
- ↑ "In Vitro Activity of Gepotidacin, a Novel Triazaacenaphthylene Bacterial Topoisomerase Inhibitor, against a Broad Spectrum of Bacterial Pathogens". Antimicrobial Agents and Chemotherapy 60 (3): 1918–23. January 2016. doi:10.1128/aac.02820-15. PMID 26729499.
- ↑ "GlaxoSmithKline kick-starts first-of-its-kind late-stage antibiotic test". FierceBiotech. 28 October 2019. https://www.fiercebiotech.com/biotech/glaxosmithkline-kickstarts-first-its-kind-late-stage-antibiotic-test.
- ↑ "Microbiological Analysis from a Phase 2 Randomized Study in Adults Evaluating Single Oral Doses of Gepotidacin in the Treatment of Uncomplicated Urogenital Gonorrhea Caused by Neisseria gonorrhoeae". Antimicrobial Agents and Chemotherapy 62 (12). December 2018. doi:10.1128/AAC.01221-18. PMID 30249694.
- ↑ "In vitro activity of the novel triazaacenaphthylene gepotidacin (GSK2140944) against MDR Neisseria gonorrhoeae". The Journal of Antimicrobial Chemotherapy 73 (8): 2072–2077. August 2018. doi:10.1093/jac/dky162. PMID 29796611.
 |

