Chemistry:Grepafloxacin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Multum Consumer Information |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 50% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H22FN3O3 |
| Molar mass | 359.401 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Grepafloxacin (trade name Raxar, Glaxo Wellcome) was an oral broad-spectrum fluoroquinolone antibacterial agent used to treat bacterial infections. Grepafloxacin was withdrawn worldwide from markets in 1999,[1][2] due to its side effect of lengthening the QT interval on the electrocardiogram, leading to cardiac events and sudden death.[3]
Clinical uses
Grepafloxacin was used for treating exacerbations of chronic bronchitis caused by susceptible bacteria (e.g. Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis),[4][5][6] community-acquired pneumonia (including those, in addition to the above germs, caused by Mycoplasma pneumoniae)[7][8] gonorrhea and non-gonococcal urethritis and cervicitis (for example caused by Chlamydia trachomatis or Ureaplasma urealyticum).[9][10]
Synthesis
The preparation of quinolones bearing a substituent at position 5 is complicated by the greater electrophilic character of the 8 position. One scheme for resolving the problem consists in blocking access to position 8 by first adding a readily removable group to that center.
The scheme starts with the conversion of the carboxylic acid in (1) to its dimethyloxazoline derivative (3) by reaction with the aminomethyl propanol (2). Lithium diisopropylamide (LDA) then removes a proton from the 8 position; treatment of that anion with trimethylsilyl iodide leads to the silylated intermediate (4). A second round of LDA then generates a carbanion at the only open position; reaction with methyl iodide leads to the corresponding 5 methyl derivative (5). Treatment of that product with cesium fluoride breaks the carbon–silicon bond, removing the silyl group; aqueous acid then hydrolyzes the oxazoline to afford the free acid (6). This last intermediate is then taken on to the quinolone (9) [13] by essentially the same scheme as that used to prepare difloxacin, with the difference that the chain elongation is by means of Grignard reagent of ethyl bromoacetate. Treatment of (9) with 2-methylpiperazine proceeds by reaction at the less hindered of the two amino groups; saponification then affords grepafloxacin (10).
Stereochemistry
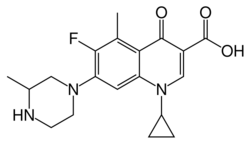
Grepafloxacin contains a stereocenter and consists of two enantiomers. This is a racemate, ie a 1: 1 mixture of (R)- and the (S)-forms:
| Enantiomers of grepafloxacin | |
|---|---|
 (R)-grepafloxacin CAS number: 146761-68-4 |
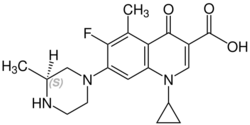 (S)-grepafloxacin CAS number: 146761-69-5 |
See also
- Quinolones
References
- ↑ "Glaxo Wellcome voluntary withdrawn Raxar (Grepafloxacin)". https://www.fda.gov/ohrms/dockets/ac/00/backgrd/3634b1a_tab5a.pdf.
- ↑ "Withdrawal of Product: RAXAR (grepafloxacin HCl) 600 mg Tablets, 400 mg Tablets, and 200 mg Tablets". U.S. Food and Drug Administration. https://www.fda.gov/ohrms/dockets/ac/00/backgrd/3634b1a_tab5b.htm.
- ↑ "Safety and tolerability of fluoroquinolones". Clinical Cornerstone Suppl 3: S29–S36. 2003. doi:10.1016/s1098-3597(03)90027-5. PMID 14992418.
- ↑ "Efficacy and safety of a 10-day course of 400 or 600 milligrams of grepafloxacin once daily for treatment of acute bacterial exacerbations of chronic bronchitis: comparison with a 10-day course of 500 milligrams of ciprofloxacin twice daily". Antimicrobial Agents and Chemotherapy 42 (1): 114–120. January 1998. doi:10.1128/AAC.42.1.114. PMID 9449270.
- ↑ "Randomized, double-blind study of grepafloxacin versus amoxycillin in patients with acute bacterial exacerbations of chronic bronchitis". The Journal of Antimicrobial Chemotherapy 40 Suppl A: 63–72. December 1997. doi:10.1093/jac/40.suppl_1.63. PMID 9484875.
- ↑ "Randomized, double-blind study of short-course (5 day) grepafloxacin versus 10 day clarithromycin in patients with acute bacterial exacerbations of chronic bronchitis". The Journal of Antimicrobial Chemotherapy 44 (4): 515–523. October 1999. doi:10.1093/jac/44.4.515. PMID 10588313.
- ↑ "Randomized, double-blind, comparative study of grepafloxacin and amoxycillin in the treatment of patients with community-acquired pneumonia". The Journal of Antimicrobial Chemotherapy 40 Suppl A: 73–81. December 1997. doi:10.1093/jac/40.suppl_1.73. PMID 9484876.
- ↑ "Respiratory pathogens: assessing resistance patterns in Europe and the potential role of grepafloxacin as treatment of patients with infections caused by these organisms". The Journal of Antimicrobial Chemotherapy 45 (90002): 1–8. March 2000. doi:10.1093/jac/45.suppl_2.1. PMID 10719006.
- ↑ "The in-vitro activity of grepafloxacin against Chlamydia spp., Mycoplasma spp., Ureaplasma urealyticum and Legionella spp". The Journal of Antimicrobial Chemotherapy 40 Suppl A: 31–34. December 1997. doi:10.1093/jac/40.suppl_1.31. PMID 9484871.
- ↑ "Daily oral grepafloxacin vs. twice daily oral doxycycline in the treatment of Chlamydia trachomatis endocervical infection". Infectious Diseases in Obstetrics and Gynecology 6 (3): 109–115. 1998. doi:10.1155/S1064744998000210. PMID 9785106.
- ↑ "Synthesis and biological activity of 5-alkyl-1,7,8-trisubstituted-6-fluoroquinoline-3-carboxylic acids". Journal of Medicinal Chemistry 34 (3): 1155–1161. March 1991. doi:10.1021/jm00107a040. PMID 2002456.
- ↑ WO patent 8906649; eidem, U.S. Patent 4,920,120 (1989, 1990 both to Warner-Lambert).
- ↑ "Synthesis of 5-methyl-4-oxo-quinolinecarboxylic acids". Journal of Heterocyclic Chemistry 27 (6): 1609–1616. 1990. doi:10.1002/jhet.5570270616.
 |


