Chemistry:Cethromycin
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Between 35.8 and 60% in animal studies. |
| Metabolism | Liver |
| Elimination half-life | 1.6, 3.0, 4.5, 5.9 and 6 hours. Mouse, Monkey, Rat, Dog and Human respectively. |
| Excretion | 7.0% urine 87.2% faeces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
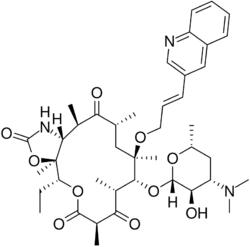
| Formula | C42H59N3O10 |
| Molar mass | 765.945 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 211 to 213 °C (412 to 415 °F) |
| |
| |
| | |
Cethromycin, trade name Restanza (initially known as ABT-773[1][2]) is a ketolide antibiotic undergoing research for the treatment of community acquired pneumonia (CAP)[1][3][4][5] and for the prevention of post-exposure inhalational anthrax, and was given an "orphan drug" status for this indication.[6] Originally discovered and developed by Abbott, it was acquired by Advanced Life Sciences Inc. for further development.
On October 1, 2008 Advanced Life Sciences submitted a New Drug Application (NDA) to Food and Drug Administration (FDA) for cethromycin to treat mild-to-moderate community acquired pneumonia.[7]
On December 3, 2008 Advanced Life Sciences announced that this New Drug Application has been accepted for filing by the FDA.[8]
In June 2009, an FDA Anti-Infective Drugs Advisory Committee review found insufficient evidence for cethromycin efficacy in treatment of community acquired pneumonia, as the Phase 3 clinical trial followed standards that were updated after the clinical trial but three months prior to review. The committee did, however, find the drug safe to use.[9]
References
- ↑ 1.0 1.1 Lawrence LE (June 2001). "ABT-773 (Abbott Laboratories)". Current Opinion in Investigational Drugs 2 (6): 766–72. PMID 11572654.
- ↑ "ABT-773: a new ketolide antibiotic". Expert Opinion on Investigational Drugs 10 (2): 343–51. February 2001. doi:10.1517/13543784.10.2.343. PMID 11178346.
- ↑ "Ketolides: an emerging treatment for macrolide-resistant respiratory infections, focusing on S. pneumoniae". Expert Opinion on Emerging Drugs 8 (2): 297–321. November 2003. doi:10.1517/14728214.8.2.297. PMID 14661991.
- ↑ Reinert RR (June 2004). "Clinical efficacy of ketolides in the treatment of respiratory tract infections". The Journal of Antimicrobial Chemotherapy 53 (6): 918–27. doi:10.1093/jac/dkh169. PMID 15117934.
- ↑ "Use of cethromycin, a new ketolide, for treatment of community-acquired respiratory infections". Expert Opinion on Investigational Drugs 17 (3): 387–400. March 2008. doi:10.1517/13543784.17.3.387. PMID 18321237.
- ↑ Cethromycin – Advanced Life Sciences
- ↑ Cethromycin New Drug Application
- ↑ Cethromycin New Drug Application accepted for filing by FDA[yes|permanent dead link|dead link}}]
- ↑ Advanced Life Sciences Holdings, Inc.. "Complete Response Letter for Restanza NDA". Drugs.com. https://www.drugs.com/nda/restanza_090806.html. Retrieved 2014-03-26.
Further reading
 |
