Chemistry:Sarecycline
 | |
| Clinical data | |
|---|---|
| Pronunciation | sar"e sye' kleen |
| Trade names | Seysara |
| Other names | P-005672 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618068 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
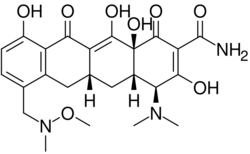
| Formula | C24H29N3O8 |
| Molar mass | 487.509 g·mol−1 |
| 3D model (JSmol) | |
| |
Sarecycline, sold under the brand name Seysara, is a narrow-spectrum tetracycline-derived antibiotic medication.[2][3] It is specifically designed for the treatment of acne, and was approved by the FDA in October 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years of age and older.[1] Two randomized and well-controlled clinical trials reported efficacy data on both facial and truncal acne (back and chest).[4] Efficacy was assessed in a total of 2002 subjects 9 years of age and older.[1] Unlike other tetracycline-class antibiotics, sarecycline has a long C7 moiety that extends into and directly interact with the bacterial messenger RNA (mRNA).[5] The spectrum of activity is limited to clinically relevant Gram-positive bacteria, mainly Cutibacterium acnes, with little or no activity against Gram-negative bacterial microflora commonly found in the human gastrointestinal tract.[6]
Medical uses
Sarecycline, is indicated for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris.[1]
References
- ↑ 1.0 1.1 1.2 1.3 "Seysara- sarecycline hydrochloride tablet, coated". DailyMed. U.S. National Library of Medicine. 1 August 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b200957c-3004-4988-be97-9fd619a83649.
- ↑ "Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris". Antimicrobial Agents and Chemotherapy 63 (1). January 2019. doi:10.1128/AAC.01297-18. PMID 30397052.
- ↑ "Sarecycline". PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/54681908.
- ↑ "Once-Daily Oral Sarecycline 1.5 mg/kg/day Is Effective for Moderate to Severe Acne Vulgaris: Results from Two Identically Designed, Phase 3, Randomized, Double-Blind Clinical Trials". Journal of Drugs in Dermatology 17 (9): 987–996. September 2018. PMID 30235387.
- ↑ "Sarecycline interferes with tRNA accommodation and tethers mRNA to the 70S ribosome". Proceedings of the National Academy of Sciences of the United States of America 117 (34): 20530–20537. August 2020. doi:10.1073/pnas.2008671117. PMID 32817463. Bibcode: 2020PNAS..11720530B.
- ↑ "Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris". Antimicrobial Agents and Chemotherapy 63 (1). January 2019. doi:10.1128/AAC.01297-18. PMID 30397052.
 |

