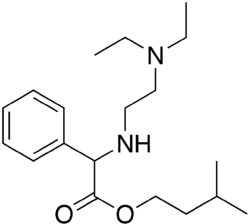
Chemistry:Camylofin
 | |
| Clinical data | |
|---|---|
| Other names | Acamylophenine |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H32N2O2 |
| Molar mass | 320.477 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Camylofin is an antimuscarinic drug.[1]
Camylofin is a smooth muscle relaxant with both anticholinergic action as well as direct smooth muscle action. Anticholinergic action is produced by inhibiting the binding of acetylcholine to muscarinic receptors, but the action is less pronounced.[citation needed] Direct smooth muscle relaxation is achieved by inhibiting phosphodiesterase type IV, which leads to increased cyclic AMP and eventually reduced cytosolic calcium. Thus camylofin has a comprehensive action to relieve smooth muscle spasm. It is used to treat stomach ache in infants and children. Usually it is given in combination with paracetamol to treat stomach ache, as well as pyrexia.[2]
Synthesis
The Hell–Volhard–Zelinsky halogenation on phenylacetic acid [103-82-2] (1) gives 2-Bromo-2-phenylacetyl bromide, CID:15621041 (2). Treatment with isoamyl alcohol [123-51-3] gives 3-methylbutyl bromo(phenyl)acetate [92018-48-9] (3). Alkylation with N,N-Diethylethylenediamine [100-36-7] (4) completed the synthesis of Camylofin (5).
References
- ↑ "Camylofin". PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/5902.
- ↑ "To compare the effect of camylofin dihydrochloride (anafortin) with combination of valethamate bromide (epidosin) and hyoscine butyl-N-bormide (buscopan) on cervical dilation". Journal of Clinical and Diagnostic Research 7 (9): 1897–9. September 2013. doi:10.7860/JCDR/2013/6231.3345. PMID 24179892.
- ↑ Bruzzese, Tiberio; Crescenzi, Elda (1966). "N-Aminoalkyl-α-aminoacids and Their Corresponding Ethyl Esters". Journal of Pharmaceutical Sciences. 55 (7): 737–740. doi:10.1002/jps.2600550717.
- ↑ Szarvasi, E. et al, Bull. Soc. Chim. Fr., 1957, 1019.
- ↑ Brock Norbert, Kuhas Engelbert, & Schmeisser Martin, U.S. Patent 2,665,300 (1954 to Asta Medica AG).
- ↑ Martin Dr-Chem Schmeisser, Engelbert Dr Phil Kuehas, Norbert Dr Med Brock, DE842206 (1952 to Asta Werke Ag Chem Fab).
- ↑ , GB688331 (1953 to Asta Medica AG).
- ↑ , GB782068 (1957 to Asta Medica AG).
- ↑ Kuhas Engelbert, Brock Norbert, & Arnold Herbert, CA566251 (1958 to Asta Medica AG).
- ↑ Arnold Herbert, Kuhas Engelbert, & Brock Norbert, U.S. Patent 2,857,395 (1958).
 |


