Chemistry:Revefenacin
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Yupelri |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619009 |
| License data |
|
| Routes of administration | Inhalation |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
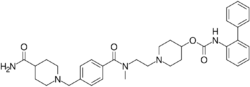
| Formula | C35H43N5O4 |
| Molar mass | 597.760 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Revefenacin, sold under the brand name Yupelri, is a medication for the treatment of chronic obstructive pulmonary disease (COPD). It was approved for use in the United States in 2018.[1] It was developed by Theravance Biopharma and is marketed by Mylan. Revefenacin is formulated as a solution that is nebulized and inhaled.[2]
Revefenacin is a bronchodilator that exerts its effect as a long-acting muscarinic antagonist.[3]
References
- ↑ "Theravance Biopharma and Mylan Receive FDA Approval for Yupelri (revefenacin) in Adults with Chronic Obstructive Pulmonary Disease" (Press release). Mylan. November 9, 2018. Archived from the original on 15 September 2019. Retrieved 17 January 2019.
- ↑ "Revefenacin: First Global Approval". Drugs 79 (1): 85–91. January 2019. doi:10.1007/s40265-018-1036-x. PMID 30560478.
- ↑ "Efficacy of revefenacin, a long-acting muscarinic antagonist for nebulized therapy, in patients with markers of more severe COPD: a post hoc subgroup analysis". BMC Pulmonary Medicine 20 (1): 134. May 2020. doi:10.1186/s12890-020-1156-4. PMID 32393215.
External links
- "Revefenacin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/revefenacin.
 |

