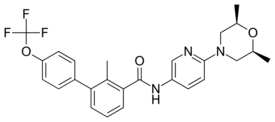
Chemistry:Sonidegib
 | |
| Clinical data | |
|---|---|
| Trade names | Odomzo |
| Other names | LDE225, erismodegib |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a615034 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Antineoplastic agents |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | <10% |
| Protein binding | >97% |
| Metabolism | Liver (CYP3A) |
| Elimination half-life | ~28 days |
| Excretion | Feces (~70%), urine (30%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H26F3N3O3 |
| Molar mass | 485.507 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sonidegib (INN), sold under the brand name Odomzo, is a medication used to treat cancer.[1]
Sonidegib is Hedgehog signaling pathway inhibitor (via smoothened antagonism).[4][5]
Approvals and indications
It was approved for medical use in the United States and in the European Union in 2015[6][1][7][8]
It is indicated for the treatment of adults with locally advanced basal-cell carcinoma that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy.[1]
Pharmacology
Sonidegib is administered by mouth. Common side effects include muscle spasms, hair loss, fatigue, abdominal pain, nausea, headache, and weight loss.[1]
Sonidegib binds to and inhibits smoothened to inhibit activation of the Hedgehog pathway. Sonidegib is primarily metabolized by CYP3A and is eliminated hepatically.[1]
Development
It has been investigated as a potential treatment for:
- Pancreatic cancer[9][10][11][12]
- Breast cancer[13][14]
- Basal cell carcinoma of the skin[15][16][17]
- Small cell lung cancer[18]
- Medulloblastoma[19][20]
- Advanced solid tumors (including ovarian, breast, pancreatic, stomach, oesophageal cancers and glioblastoma multiforme)[21][22][23]
- Acute leukemia[24] and chronic myeloid leukemia[25]
- Myelofibrosis and essential thrombocythaemia[26]
It has demonstrated significant efficacy against melanoma in vitro and in vivo.[27] It also demonstrated efficacy in a mouse model of pancreatic cancer.[28]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Odomzo- sonidegib capsule". 29 May 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=028312dc-d155-4fd5-8abd-6bb9f011d3cc.
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2015". 21 June 2022. https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia-2015.
- ↑ "Summary Basis of Decision (SBD) for Odomzo". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00490&lang=en.
- ↑ "LDE225 - PubChem". PubChem. National Institutes of Health. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=135626837.
- ↑ "Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist". ACS Medicinal Chemistry Letters 1 (3): 130–4. June 2010. doi:10.1021/ml1000307. PMID 24900187.
- ↑ "FDA approves new treatment for most common form of advanced skin cancer". https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm455862.htm.
- ↑ "FDA approves Novartis's advanced skin cancer drug". 24 July 2015. https://news.yahoo.com/fda-approves-novartiss-advanced-skin-cancer-drug-162113308--finance.html.
- ↑ "Odomzo". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/odomzo.
- ↑ "A Biomarker Study to Identify Predictive Signatures of Response to LDE225 (Hedgehog Inhibitor) In Patients With Resectable Pancreatic Cancer". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/show/NCT01911416.
- ↑ "Gemcitabine + Nab-paclitaxel With LDE-225 (Hedgehog Inhibitor) as Neoadjuvant Therapy for Pancreatic Adenocarcinoma". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01431794.
- ↑ "Dose-escalation, and Safety Study of LDE225 and Gemcitabine in Locally Advanced or Metastatic Pancreatic Cancer Patients". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT02027376.
- ↑ "A Pilot Study of a Hedgehog Pathway Inhibitor (LDE-225) in Surgically Resectable Pancreas Cancer". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01694589.
- ↑ "Study With LDE225 in Combination With Docetaxel in Triple Negative (TN) Advanced Breast Cancer (ABC) Patients (EDALINE)". ClinicalTrials.gov (National Institutes of Health). 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT02027376.
- ↑ "LDE225 in Treating Patients With Stage II-III Estrogen Receptor- and HER2-Negative Breast Cancer". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01757327.
- ↑ "A Phase II Study of Efficacy and Safety in Patients With Locally Advanced or Metastatic Basal Cell Carcinoma (BOLT)". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01327053.
- ↑ "To Evaluate the Safety, Local Tolerability, PK and PD of LDE225 on Sporadic Superficial and Nodular Skin Basal Cell Carcinomas(sBCC)". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01033019.
- ↑ "A Trial to Evaluate the Safety, Local Tolerability, Pharmacokinetics and Pharmacodynamics of LDE225 on Skin Basal Cell Carcinomas in Gorlin Syndrome Patients". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT00961896.
- ↑ "Combination of the Hedgehog Inhibitor, LDE225, With Etoposide and Cisplatin in the First-Line Treatment of Patients With Extensive Stage Small Cell Lung Cancer (ES-SCLC)". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01579929.
- ↑ "A Phase III Study of Oral LDE225 Versus (vs) Temozolomide (TMZ) in Patients With Hedge-Hog (Hh)-Pathway Activated Relapsed Medulloblastoma (MB)". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01708174.
- ↑ "A Phase I Dose Finding and Safety Study of Oral LDE225 in Children and a Phase II Portion to Assess Preliminary Efficacy in Recurrent or Refractory MB". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01125800.
- ↑ "Phase Ib, Dose Escalation Study of Oral LDE225 in Combination With BKM120 in Patients With Advanced Solid Tumors". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01576666.
- ↑ "Dose Finding and Safety of Oral LDE225 in Patients With Advanced Solid Tumors". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT00880308.
- ↑ "LDE225 and Paclitaxel in Solid Tumors". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01954355.
- ↑ "Study of Efficacy and Safety of LDE225 in Adult Patients With Relapsed/Refractory Acute Leukemia". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01826214.
- ↑ "Nilotinib and LDE225 in the Treatment of Chronic or Accelerated Phase Myeloid Leukemia in Patients Who Developed Resistance to Prior Therapy". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01456676.
- ↑ "A Phase Ib/II Dose-finding Study to Assess the Safety and Efficacy of LDE225 + INC424 in Patients With MF". ClinicalTrials.gov. National Institutes of Health. 13 February 2014. http://clinicaltrials.gov/ct2/show/NCT01787552.
- ↑ "NVP-LDE225, a potent and selective SMOOTHENED antagonist reduces melanoma growth in vitro and in vivo". PLOS ONE 8 (7): e69064. 30 July 2013. doi:10.1371/journal.pone.0069064. PMID 23935925. Bibcode: 2013PLoSO...869064J.
- ↑ "Hedgehog inhibition with the orally bioavailable Smo antagonist LDE225 represses tumor growth and prolongs survival in a transgenic mouse model of islet cell neoplasms". Annals of Surgery 254 (5): 818–23; discussion 823. November 2011. doi:10.1097/SLA.0b013e318236bc0f. PMID 22042473.
External links
- "Sonidegib". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/sonidegib.
- "Sonidegib phosphate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/sonidegib%20phosphate.
 |

