Chemistry:Trametinib
 | |
| Clinical data | |
|---|---|
| Trade names | Mekinist, Spexotras |
| Other names | GSK1120212 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613040 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
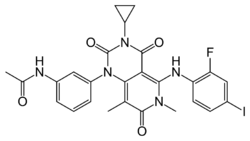
| Formula | C26H23FIN5O4 |
| Molar mass | 615.404 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trametinib, sold under the brand name Mekinist among others, is an anticancer medication used for the treatment of melanoma[2][3] and glioma.[4][5] It is a MEK inhibitor drug with anti-cancer activity.[6] It inhibits MEK1 and MEK2.[6] It is taken by mouth.[2][3]
The most common side effects include rash, diarrhea, tiredness, peripheral edema (swelling, especially of ankles and feet), nausea and acneiform dermatitis (acne-like inflammation of the skin).[3] When taken in combination with dabrafenib the most common side effects include fever, tiredness, nausea, chills, headache, diarrhea, vomiting, joint pain and rash.[3]
In May 2013, trametinib was approved as a single-agent by the US Food and Drug Administration for the treatment of people with V600E mutated metastatic melanoma.[7][8] It was approved for medical use in the European Union in June 2014.[3]
Medical uses
Trametinib, as monotherapy or in combination with dabrafenib is indicated for the treatment of melanoma and glioma.[2][3][4][5]
History
Clinical trial data demonstrated that resistance to single-agent trametinib often occurs within 6 to 7 months.[9] To overcome this, trametinib was combined with the BRAF inhibitor dabrafenib.[9] As a result of this research, on 8 January 2014, the FDA approved the combination of dabrafenib and trametinib for the treatment of patients with BRAF V600E/K-mutant metastatic melanoma.[10] On 1 May 2018, the FDA approved the combination dabrafenib/trametinib as an adjuvant treatment for BRAF V600E-mutated, stage III melanoma after surgical resection based on the results of the COMBI-AD phase 3 study,[11] making it the first oral chemotherapy regimen that prevents cancer relapse for node positive, BRAF-mutated melanoma.[12]
Society and culture
Legal status
In November 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Spexotras, intended for the treatment of low- and high-grade glioma (LGG and HGG).[13] The applicant for this medicinal product is Novartis Europharm Limited.[13] Spexotras was approved for medical use in the European Union in January 2024.[4][5]
Research
Trametinib had good results for metastatic melanoma carrying the BRAF V600E mutation in a phase III clinical trial. In this mutation, the amino acid valine (V) at position 600 within the BRAF protein has become replaced by glutamic acid (E) making the mutant BRAF protein constitutively active.[14]
Trametinib has been used off label to treat various RASopathies, including Noonan Syndrome and Primary Intestinal Lymphangiectasia.[15][16][17]
References
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2014". 21 June 2022. https://www.tga.gov.au/resources/resource/guidance/prescription-medicines-registration-new-chemical-entities-australia-2014.
- ↑ 2.0 2.1 2.2 2.3 "Mekinist- trametinib tablet, film coated". 22 June 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0002ad27-779d-42ab-83b5-bc65453412a1.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Mekinist EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/mekinist. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 4.0 4.1 4.2 4.3 "Spexotras Product information". 8 January 2024. https://ec.europa.eu/health/documents/community-register/html/h1781.htm.
- ↑ 5.0 5.1 5.2 5.3 "Spexotras EPAR". 9 November 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/spexotras.
- ↑ 6.0 6.1 "Trametinib". NCI Drug Dictionary. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/trametinib-dimethyl-sulfoxide.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Drug Approval Package: Mekinist (trametinib) Tablets NDA #204114". 8 July 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204114Orig1s000TOC.cfm.
- ↑ "GSK melanoma drugs add to tally of U.S. drug approvals". Reuters. 30 May 2013. https://www.reuters.com/article/us-glaxosmithkline-approvals-idUSBRE94S1A020130530.
- ↑ 9.0 9.1 "Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations". The New England Journal of Medicine 367 (18): 1694–703. November 2012. doi:10.1056/NEJMoa1210093. PMID 23020132.
- ↑ "Dabrafenib/Trametinib Combination Approved for Advanced Melanoma". OncLive. 9 January 2014. http://www.onclive.com/web-exclusives/FDA-Approves-First-Ever-Combination-for-Metastatic-Melanoma.
- ↑ "Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma". The New England Journal of Medicine 377 (19): 1813–1823. November 2017. doi:10.1056/NEJMoa1708539. PMID 28891408. https://espace.library.uq.edu.au/view/UQ:697684/UQ697684_OA.pdf. Retrieved 1 October 2019.
- ↑ "FDA Approves Adjuvant Combo for BRAF+ Melanoma". WebMD LLC. https://www.medscape.com/viewarticle/895984.
- ↑ 13.0 13.1 "Spexotras: Pending EC decision". 10 November 2023. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/spexotras. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "METRIC phase III study: Efficacy of trametinib (T), a potent and selective MEK inhibitor (MEKi), in progression-free survival (PFS) and overall survival (OS), compared with chemotherapy (C) in patients (pts) with BRAFV600E/K mutant advanced or metastatic melanoma (MM)". Journal of Clinical Oncology 30 (18_suppl): LBA8509. 31 January 2017. doi:10.1200/jco.2012.30.18_suppl.lba8509.
- ↑ Leegaard, Anne; Gregersen, Pernille A.; Nielsen, Trine Ø.; Bjerre, Jesper V.; Handrup, Mette M. (November 2022). "Succesful [sic] MEK-inhibition of severe hypertrophic cardiomyopathy in RIT1-related Noonan Syndrome" (in en). European Journal of Medical Genetics 65 (11): 104630. doi:10.1016/j.ejmg.2022.104630. PMID 36184070.
- ↑ Bergqvist, Christina; Wolkenstein, Pierre (March 2021). "MEK inhibitors in RASopathies" (in en). Current Opinion in Oncology 33 (2): 110–119. doi:10.1097/CCO.0000000000000711. ISSN 1040-8746. PMID 33395032. https://journals.lww.com/10.1097/CCO.0000000000000711.
- ↑ PIL, Unraveling Adult-Onset. "Unraveling Adult-Onset PIL". http://lymphangiectasia.com/.
 |

