Chemistry:Neratinib
 | |
| Clinical data | |
|---|---|
| Trade names | Nerlynx, Hernix |
| Other names | HKI-272 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a617034 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Antineoplastic agent |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
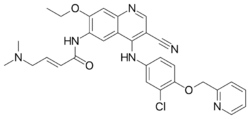
| Formula | C30H29ClN6O3 |
| Molar mass | 557.05 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Neratinib (INN), sold under the brand name Nerlynx, is a tyrosine kinase inhibitor anti-cancer medication used for the treatment of breast cancer.[3][4]
The most common side effect is diarrhea, which affects nearly all patients.[4] Other common side effects include nausea (feeling sick), vomiting, tiredness, belly pain, rash, decreased appetite, stomatitis (sore, inflamed mouth), and muscle spasms.[4]
Medical uses
In the European Union and the United States, neratinib is indicated for the extended adjuvant treatment of adults with early stage hormone receptor positive HER2-overexpressed/amplified breast cancer and who are less than one year from the completion of prior adjuvant trastuzumab based therapy.[3][4]
In the United States, it is also indicated, in combination with capecitabine, for the treatment of adults with advanced or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens in the metastatic setting.[3]
Contraindications
Women who are pregnant should not take it, and women should not become pregnant while taking it, and women who are breast-feeding should not use it, as it can cause harm to the fetus and to the baby.[3]
Adverse effects
Neratinib can cause life-threatening diarrhea in some people and mild to moderate diarrhea in almost everyone; people who take it are also at risk for complications of diarrhea like dehydration and electrolyte imbalance.[3] Similarly, there is a risk of severe liver damage and many patients have some level of it; symptoms of liver damage include fatigue, nausea, vomiting, right upper quadrant pain or tenderness, fever, rash, and high levels of eosinophils.[3]
In addition to the above, more than 10% of people taking it have nausea, abdominal pain, vomiting, sores on their lips, stomach upset, decreased appetite, rashes, and muscle spasms.[3]
Interactions
People taking neratinib should not also take gastric acid reducing agents including proton pump inhibitors and H2-receptor antagonists; antacids may be used three hours before of after taking it.[3]
Drugs that inhibit CYP3A4 increase the activity of neratinib and can make adverse effects worse, and drugs that induce CYP3A4 make neratinib less active and can reduce its efficacy. Neratinib also inhibits p-glycoprotein and effectively raises the dose of drugs like digoxin that depend on it for elimination.[3]
Pharmacology
Like lapatinib and afatinib, it is a dual inhibitor of the human epidermal growth factor receptor 2 (Her2) and epidermal growth factor receptor (EGFR) kinases.[5][6] It inhibits them by covalently binding with a cysteine side chain in those proteins.[7] Unlike related noncovalent inhibitors, neratinib is effective against the T790M resistant variant of EGFR.[8]
Neratinib has an IC50 of 59 nM against HER2 and shows weak inhibition against KDR and Scr with IC50 values of 0.8 μM and 1.4 μM, respectively. In BT474 cells, neratinib reduces HER2 autophosphorylation, and inhibited cyclin D1 expression while reduced proliferation has been observed A431 cells when treated with neratinib at concentrations of 3 or 5 nM.[9] In xenograft models with 3T3/neu tumors oral administration of neratinib at 10, 20, 40 or 80 mg/kg was able to inhibit tumor growth while in SK-OV-3 models doses of 5 and 60 mg/kg significantly inhibited tumor growth.[10]
Cell Biology
Neratinib is found to strongly reduce the amount of HER2 released by extracellular vescicles and to enhance the capacity of clathrin mediated endocytosis. However, despite HER2 mediated signaling downregulation, Neratinib exerts only a modest effect on HER2 trafficking at IC50 of 6nM in SKBR3 cells. [11]
Chemistry
Neratinib is a 4-anilino-3-cyano quinoline derivative.[3]
History
Neratinib was discovered and initially developed by Wyeth; Pfizer continued development up to Phase III in breast cancer, and licensed it to Puma Biotechnology in 2011.[12]
It was approved for medical use in the United States in July 2017, for the extended adjuvant treatment of adults with early stage HER2-overexpressed/amplified breast cancer, (after adjuvant trastuzumab-based therapy).[13][14][15] Approval was based on the ExteNET trial (NCT00878709), a multicenter, randomized, double-blind, placebo-controlled trial of neratinib following adjuvant trastuzumab treatment.[14][15][16]
Neratinib was approved for medical use in the European Union in August 2018.[4]
Brand names
In Bangladesh it is sold under the trade name Hernix.[17]
References
- ↑ "Summary Basis of Decision (SBD) for Nerlynx". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00461&lang=en.
- ↑ "Nerlynx 40 mg Film-coated Tablets - Summary of Product Characteristics (SmPC)". https://www.medicines.org.uk/emc/product/10477.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 "Nerlynx- neratinib tablet". 6 August 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d1e41dcf-e82a-47c2-a0ad-6c6eef621834.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 "Nerlynx EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/nerlynx. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Advances in the management of HER2-positive early breast cancer". Critical Reviews in Oncology/Hematology 119: 113–122. November 2017. doi:10.1016/j.critrevonc.2017.10.001. PMID 29042085.
- ↑ "The major lung cancer-derived mutants of ERBB2 are oncogenic and are associated with sensitivity to the irreversible EGFR/ERBB2 inhibitor HKI-272". Oncogene 26 (34): 5023–5027. July 2007. doi:10.1038/sj.onc.1210292. PMID 17311002.
- ↑ "The resurgence of covalent drugs". Nature Reviews. Drug Discovery 10 (4): 307–317. April 2011. doi:10.1038/nrd3410. PMID 21455239.
- ↑ "The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP". Proceedings of the National Academy of Sciences of the United States of America 105 (6): 2070–2075. February 2008. doi:10.1073/pnas.0709662105. PMID 18227510. Bibcode: 2008PNAS..105.2070Y.
- ↑ "Neratinib shows efficacy in the treatment of HER2 amplified carcinosarcoma in vitro and in vivo". Gynecologic Oncology 139 (1): 112–117. October 2015. doi:10.1016/j.ygyno.2015.08.002. PMID 26260909.
- ↑ "New mouse xenograft model modulated by tumor-associated fibroblasts for human multi-drug resistance in cancer". Oncology Reports 34 (5): 2699–2705. November 2015. doi:10.3892/or.2015.4265. PMID 26352907.
- ↑ "Imaging of Endocytic Trafficking and Extracellular Vesicles Released Under Neratinib Treatment in ERBB2+ Breast Cancer Cells". The Journal of Histochemistry and Cytochemistry 69 (7): 461–473. July 2021. doi:10.1369/00221554211026297. PMID 34126793.
- ↑ "Puma Acquires Global Rights to Pfizer's Phase III Breast Cancer Drug Neratinib". GEN. October 6, 2011. https://www.genengnews.com/gen-news-highlights/puma-acquires-global-rights-to-pfizer-s-phase-iii-breast-cancer-drug-neratinib/81245787/.
- ↑ "Nerlynx (neratinib maleate) Tablets". 21 August 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208051Orig1s000TOC.cfm.
- ↑ 14.0 14.1 "FDA approves new treatment to reduce the risk of breast cancer returning". U.S. Food and Drug Administration (FDA) (Press release). 17 July 2017. Retrieved 13 November 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 15.0 15.1 "FDA approves neratinib for extended adjuvant treatment of early stage HER2-positive breast cancer". 17 July 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neratinib-extended-adjuvant-treatment-early-stage-her2-positive-breast-cancer.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Drug Trials Snapshot: Nerlynx". 17 July 2017. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshot-nerlynx.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Hernix". medex.com.bd. https://medex.com.bd/brands/27301/hernix-40mg.
External links
- "Neratinib". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/neratinib.
- "Neratinib maleate". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/neratinib%20maleate.
- "Neratinib maleate". 27 July 2017. https://www.cancer.gov/about-cancer/treatment/drugs/neratinibmaleate.
- "Neratinib maleate". NCI Drug Dictionary. National Cancer Institute. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/neratinib.
- Clinical trial number NCT00878709 for "Study Evaluating The Effects Of Neratinib After Adjuvant Trastuzumab In Women With Early Stage Breast Cancer (ExteNET)" at ClinicalTrials.gov
 |
