Chemistry:Dacomitinib
 | |
| Clinical data | |
|---|---|
| Pronunciation | dak" oh mi' ti nib |
| Trade names | Vizimpro |
| Other names | PF-00299804 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618055 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% |
| Protein binding | 98% |
| Metabolism | CYP2D6, CYP3A4 |
| Metabolites | O-desmethyl-dacomitinib |
| Elimination half-life | 70 hrs |
| Excretion | 79% faeces, 3% urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
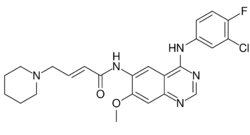
| Formula | C24H25ClFN5O2 |
| Molar mass | 469.95 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dacomitinib, sold under the brand name Vizimpro, is a medication for the treatment of non-small-cell lung carcinoma (NSCLC). It is a selective and irreversible inhibitor of EGFR.[3]
Dacomitinib has advanced to several Phase III clinical trials.[when?] The January 2014 results of the first trials were disappointing, with a failure to meet the study goals.[4][5][6] Additional Phase III trials are ongoing[when?].[4]
In 2017, results of a trial comparing dacomitinib to gefitinib for NSCLC (driven by mutated EGFR) were announced.[7]
Dacomitinib was approved for medical use in the United States in September 2018,[8] in Japan in 2019, and in the European Union in 2019,[9] for the treatment of non-small cell lung cancer with epidermal growth factor receptor (EGFR) gene mutation.
References
- ↑ "Vizimpro Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=97625.
- ↑ "Summary Basis of Decision (SBD) for Verzenio". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00447&lang=en.
- ↑ "Dacomitinib". NCI Drug Dictionary. National Cancer Institute, U.S. Department of Health and Human Services. http://www.cancer.gov/drugdictionary?cdrid=462567.
- ↑ 4.0 4.1 Chustecka, Zosia (January 27, 2014). "Dacomitinib Fails in Pretreated Non-small Cell Lung Cancer". Medscape. http://www.medscape.com/viewarticle/819761.
- ↑ Taylor, Phil (28 January 2014). "Blow to Pfizer as dacomitinib fails in lung cancer trials". pmlive.com. http://www.pmlive.com/pharma_news/blow_to_pfizer_as_dacomitinib_fails_in_lung_cancer_trials_537815.
- ↑ "Pfizer Announces Top-Line Results From Two Phase 3 Trials Of Dacomitinib In Patients With Refractory Advanced Non-Small Cell Lung Cancer". Pfizer Press Release. January 27, 2014. http://www.pfizer.com/news/press-release/press-release-detail/pfizer_announces_top_line_results_from_two_phase_3_trials_of_dacomitinib_in_patients_with_refractory_advanced_non_small_cell_lung_cancer.
- ↑ Smith, Michael (6 June 2017). "Dacomitinib Sets PFS Record in Phase III NSCLC Trial". MedPage Today. https://www.medpagetoday.com/MeetingCoverage/ASCO/65818.
- ↑ "Dacomitinib: First Global Approval". Drugs 78 (18): 1947–1953. December 2018. doi:10.1007/s40265-018-1028-x. PMID 30506139.
- ↑ "Vizimpro EPAR". 5 June 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/vizimpro.
External links
- "Dacomitinib". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/dacomitinib.
 |
