Chemistry:Vismodegib
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌvɪsmoʊˈdɛɡɪb/ VIS-moh-DEG-ib |
| Trade names | Erivedge |
| Other names | GDC-0449, RG-3616 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 31.8% |
| Protein binding | >99% |
| Metabolism | <2% metabolised by CYP2C9, CYP3A4, CYP3A5 |
| Elimination half-life | 4 days (continuous use), 12 days (single dose) |
| Excretion | Fecal (82%), Urinary (4.4%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
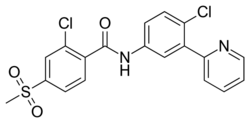
| Formula | C19H14Cl2N2O3S |
| Molar mass | 421.29 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vismodegib, sold under the brand name Erivedge, is a medication used for the treatment of basal-cell carcinoma (BCC).[2] The approval of vismodegib on January 30, 2012, represents the first Hedgehog signaling pathway targeting agent to gain U.S. Food and Drug Administration (FDA) approval.[3] The drug is also undergoing clinical trials for metastatic colorectal cancer, small-cell lung cancer, advanced stomach cancer, pancreatic cancer, medulloblastoma and chondrosarcoma as of June 2011[update].[4] The drug was developed by the biotechnology/pharmaceutical company Genentech.[3]
Indication
Vismodegib is indicated for people with basal-cell carcinoma (BCC) which has metastasized to other parts of the body, relapsed after surgery, or cannot be treated with surgery or radiation.[3][5]
Mechanism of action
The substance acts as a cyclopamine-competitive antagonist of the smoothened receptor (SMO) which is part of the Hedgehog signaling pathway.[4] SMO inhibition causes the transcription factors GLI1 and GLI2 to remain inactive, which prevents the expression of tumor mediating genes within the hedgehog pathway.[6] This pathway is pathogenetically relevant in more than 90% of basal-cell carcinomas.[7]
Side effects
In clinical trials, common side effects included gastrointestinal disorders (nausea, vomiting, diarrhoea, constipation), muscle spasms, fatigue, hair loss, and dysgeusia (distortion of the sense of taste).[2]
Development
Vismodegib has undergone several promising phase I and phase II clinical trials for its use in treating medulloblastoma.[8]
References
- ↑ 1.0 1.1 "Erivedge® (vismodegib)". Australian Prescribing Information, Australian Register of Therapeutic Goods (ARTG). Roche Products Pty Limited. 17 November 2022. https://www.guildlink.com.au/gc/ws/ro/pi.cfm?product=roperive10615.
- ↑ 2.0 2.1 2.2 "Erivedge- vismodegib capsule". DailyMed. U.S. National Library of Medicine. 9 April 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a.
- ↑ 3.0 3.1 3.2 "FDA approves Erivedge (vismodegib) capsule, the first medicine for adults with advanced basal cell carcinoma". 30 January 2012. https://www.roche.com/investors/updates/inv-update-2012-01-30.htm.
- ↑ 4.0 4.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedProus - ↑ Targeted Therapies in Cancer. Hauppauge, NY: Nova Sciences Publishers. 2014. ISBN 978-1-63321-687-7. https://www.novapublishers.com/catalog/product_info.php?products_id=50994. Retrieved 2014-07-13.
- ↑ "Vismodegib (GDC-0449) Smoothened Inhibitor". BioOncology. Genentech. http://www.biooncology.com/pipeline-molecules/vismodegib/index.html.
- ↑ "Neue Wirkstoffe – Vismodegib" (in German). Österreichische Apothekerzeitung (14/2011): 10. 4 July 2011.
- ↑ "Phase I and phase II sonidegib and vismodegib clinical trials for the treatment of paediatric and adult MB patients: a systemic review and meta-analysis". Acta Neuropathologica Communications 7 (1): 123. July 2019. doi:10.1186/s40478-019-0773-8. PMID 31362788.
Further reading
- "Efficacy and safety of vismodegib in advanced basal-cell carcinoma". The New England Journal of Medicine 366 (23): 2171–2179. June 2012. doi:10.1056/NEJMoa1113713. PMID 22670903.
External links
- "Vismodegib". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/vismodegib.
 |
